Summary
-
1.
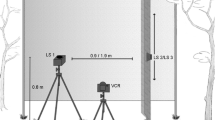
The steering responses of three species of field crickets,Teleogryllus oceanicus, T. commodus, andGryllus bimaculatus, were characterized during tethered flight using single tonepulses (rather than model calling song) presented at carrier frequencies from 3–100 kHz. This range of frequencies encompasses the natural songs of crickets (4–20 kHz, Fig. 1) as well as the echolocation cries of insectivorous bats (12–100 kHz).
-
2.
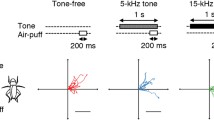
The single-pulse stimulus paradigm was necessary to assess the aversive nature of high carrier frequencies without introducing complications due to the attractive properties of repeated pulse stimuli such as model calling songs. Unlike the natural calling song, single tone-pulses were not attractive and did not elicit positive phonotactic steering even when presented at the calling song carrier frequency (Figs. 2, 3, and 9). In addition to temporal pattern, phonotactic steering was sensitive to carrier frequency as well as sound intensity. Three discrete flight steering behaviors (1)positive phonotaxis, (2)negative phonotaxis and (3)evasion, were elicited by appropriate combinations of frequency, temporal pattern and sound intensity (Fig. 12). Positive phonotactic steering required a model calling song temporal pattern, was tuned to 5 kHz and was restricted to frequencies below 9 kHz. Negative phonotactic steering, similar to the ‘early warning’ bat-avoidance behavior of moths, was produced by low intensity (55 dB SPL) tone-pulses at frequencies between 12 and 100 kHz (Figs. 2, 3, and 9). In contrast to model calling song, single tone-pulses of high intensity 5–10 kHz elicited negative phonotactic steering; low intensity ultrasound (20–100 kHz) produced only negative phonotactic steering, regardless of pulse repetition pattern. ‘Evasive’, side-to-side steering, similar to the ‘last-chance’ bat-evasion behavior of moths was produced in response to high intensity (> 90 dB) ultrasound (20–100 kHz).
-
3.
Since the demonstration of negative phonotactic steering did not require the use of a calling song temporal pattern, avoidance of ultrasound cannot be the result of systematic errors in localizing an inherently attractive stimulus when presented at high carrier frequencies. Unlike attraction to model calling song, the ultrasound-mediated steering responses were of short latency (25–35 ms) and were produced in an open loop manner (Fig. 4), both properties of escape behaviors. The ultrasound-mediated steering behaviors were produced in response to a variety of synthetic acoustic stimuli resembling bat biosonar, e.g. short ultrasonic pulses (as short as 1 ms), repetition rates from 1–500 pps and frequencies from 15 to 100 kHz (Figs. 5, 6), and were strikingly similar to the bat-escape behaviors of moths.
-
4.
Like moths, flying crickets steered away from low intensity ultrasound indicative of distant bats (10–18 m away), but produced the ‘evasive’ steering in response to high intensity ultrasound indicative of a closely approaching bat at 1–2 m. Theoretical calculations of ultrasound transmission predict that crickets can detect and avoid ultrasound at distances on the order of tens of meters, well beyond the detection abilities of bats for insect-size objects (less than 5 m) (Fig. 11). The ultrasound-mediated steering behavior of flying crickets is well suited to bat-avoidance. This study demonstrates that acoustically mediated steering in flying crickets is more diverse and more complex than previously thought.
Similar content being viewed by others
Abbreviations
- SPL :
-
sound pressure level
- LRTB :
-
Left/Right Threshold Bias
- BTD :
-
Binaural Threshold Difference
- DLMs :
-
Dorsal Longitudinal Muscles
- CS :
-
calling song
- CI :
-
confidence interval
References
Bailey NTJ (1974) Statistical methods in biology. English Universities Press, London 200 pp
Bentley D, Hoy RR (1970) Postembryonic development of adult motor patterns in crickets: a neural analysis. Science 170:1409–1411
Boyan G (1981) Two-tone suppression of an identified auditory neurone in the brain of the cricketGryllus bimaculatus (De Geer). J Comp Physiol 144:117–125
Boyd P, Kühne R, Silver S, Lewis B (1984) Two-tone suppression and song coding by ascending neurons in the cricketGryllus campestris L. J Comp Physiol A 154:423–430
Cade W (1979a) The evolution of alternative male reproductive strategies in field crickets. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition in insects. Academic Press, New York, pp 343–379
Cade W (1979b) Field cricket dispersal flights measured by crickets landing at lights. Texas J Sci 31:2
Camhi J (1970) Yaw-correcting postural changes in locusts. J Exp Biol 52:519–531
Camhi J, Nolen TG (1981) Properties of the escape system of cockroaches during walking. J Comp Physiol 124:339–346
Camhi J, Tom W (1978) The escape behavior of the cockroachPeriplaneta americana I. Turning response to wind puffs. J Comp Physiol 128:193–201
Cranbrook Earl of, Barret HG (1965) Observations on noctule bats (Nyctalus noctula) captured while feeding. Proc Zool Soc Lond 144:1–24
Easterla DA, Whitaker JO (1972) Food habits of some bats from Big Bend Nat'l Park, Texas. J Mammol 53:887–890
Eaton RC, Bombardieri RA (1978) Behavioral functions of the Mauthner neuron. In: Faber D, Korn H (eds) Neurobiology of the Mauthner cell. Raven Press, New York, pp 221–243
Esch H, Huber F, Wohlers DW (1980) Primary auditory neurons in crickets: Physiology and central projections. J Comp Physiol 137:27–38
Fenton MB (1979) Adaptiveness and ecology of echolocation in terrestrial (aerial) systems. In: Busnel RG, Fish JF (eds) Animal sonar sytems. Plenum Press, New York London, pp 427–446
Fenton MB, Fullard JH (1981) Moth hearing and the feeding strategies of bats. Am Sci 69:266–275
Fenton MB, Morris GK (1976) Opportunistic feeding by desert bats (Myotis spp). Can J Zool 54:526–530
Fullard JH, Thomas DW (1982) Detection of certain African insectivorous bats by sympatric, tympanate moths. J Comp Physiol 143:363–368
Griffin DR (1958) Listening in the dark. Yale University Press, New Haven
Griffin DR, Webster FA, Michael CR (1960) The echolocation of flying insects by bats. Anim Behav 8:141–154
Griffin DR, Friend JH, Webster FA (1965) Target discrimination by the echolocation of bats. J Exp Zool 158:155–168
Hill KG, Boyan GS (1977) Sensitivity to frequency and direction of sound in the auditory system of crickets (Gryllidae). J Comp Physiol 121:79–97
Hill KG, Loftus-Hills JJ, Gartside DF (1972) Pre-mating isolation between the Australian field cricketTeleogryllus commodus andT. oceanicus (Orthoptera: Gryllidae). Aust J Zool 20:153–163
Hoy RR, Pollack GS, Moiseff A (1982) Species-recognition in the field cricket,Teleogryllus oceanicus: behavioral and neural mechanisms. Am Zool 22:597–607
Hutchings M, Lewis B (1981) Response properties of primary auditory fibers in the cricketTeleogryllus oceanicus (Le Guillou). J Comp Physiol 143:129–134
Hutchings M, Lewis B (1984) The role of two-tone suppression in song coding by ventral cord neurones in the cricket,Teleogryllus oceanicus (Le Guillou). J Comp Physiol A 154:103–112
Kick SA (1982) Target-detection by the echolocating bat,Eptesicus fuscus. J Comp Physiol 145:431–435
Kick SA, Simmons JA (1984) Automatic gain control in the bat's sonar receiver and the neuroethology of echolocation. J Neurosci 4:2725–2737
Kunz TH (1974) Feeding ecology of a temperate insectivorous bat (Myotis vilifer). Ecology 55:693–771
Larsen ON (1981) Mechanical time resolution in some insect ears II. Impulse sound transmission in acoustic tracheal tubes. J Comp Physiol 143:297–304
Lawrence BD, Simmons JA (1982) Measurements of atmospheric attenuation at ultrasonic frequencies and the significance for echolocation by bats. J Acoust Soc 71:585–590
Leroy Y (1966) Signaux acoustiques, comportement et systématique de quelques espèces de Gryllides (Orthoptères, Ensifères) THESIS, Université de Paris. Extract Du Bulletin Biologique. Tome. C. Fase. 1
Loher W, Rence B (1978) The mating behavior ofTeleogryllus commodus (Walker) and its central and peripheral control. Z Tierpsychol 46:225–259
McCue JJ (1961) Ultrasonic instrumentation for research on bats. IRE Int Conv Rec 9:310–315
Michelsen A, Larsen ON (1983) Strategies for acoustic communication in complex environments. In: Huber F, Markl H (eds) Neuroethology and behavioral physiology. Springer, Berlin Heidelberg New York, pp 321–331
Miller LA (1971) Physiological responses of lacewings (Chrysopa, Neuroptera) to ultrasound. J Insect Physiol 17:491–506
Miller LA (1975) The behavior of flying green lacewings.Chrysopa carnea, in the presence of ultrasound. J Insect Physiol 21:205–219
Miller LA (1982) The orientation and evasive behavior of insects to bat cries. In: Addink ADF, Spronk N (eds) Exogenous and endogenous influences on metabolic and neuronal control, vol 1. Pergamon Press, Oxford, pp 393–405
Miller LA (1983) How insects detect and avoid bats. In: Huber F, Markl H (eds) Neuroethology and behavioral physiology. Springer, Berlin Heidelberg New York, pp 251–266
Miller LA, Olesen J (1979) Avoidance behavior in green lacewings I. Behavior of free-flying green lacewings to hunting bats and to ultrasound. J Comp Physiol 131:113–120
Moiseff A, Hoy RR (1983) Sensitivity to ultrasound in an identified auditory interneuron in the cricket: a possible neural link to phonotactic behavior. J Comp Physiol 152:155–167
Moiseff A, Pollack GS, Hoy RR (1978) Steering responses of flying crickets to sound and ultrasound: Mate attraction and predator avoidance. Proc Natl Acad Sci USA 75:4052–4056
Nocke H (1972) Physiological aspects of sound communication in crickets (Gryllus campestris L.). J Comp Physiol 80:141–162
Nolen TG (1984) The neural basis of ultrasound avoidance steering of flying crickets. PhD Thesis, Cornell University, Ithaca, New York
Nolen TG, Hoy RR (1982) Neural basis of ultrasound avoidance in the cricketTeleogryllus oceanicus. Soc Neurosci Abstr 8:529
Nolen TG, Hoy RR (1983) Postsynaptic inhibition mediates suppression of ultrasound avoidance in the cricketTeleogryllus oceanicus. Soc Neurosci Abstr 9:215
Nolen TG, Hoy RR (1984) Initiation of behavior by single neurons: The role of behavioral context. Science 226:992–994
Nolen TG, Hoy RR (1986a) Phonotaxis in flying crickets. II. Physiological mechanisms of two-tone suppression of the high frequency avoidance steering behavior by the calling song. J Comp Physiol A 159:441–456
Nolen TG, Hoy RR (1986b) Postsynaptic inhibition mediates high frequency selectivity in the cricketTeleogryllus oceanicus: Implications for flight phonotaxis behavior. Accepted for publication in J Neurosci
Novick A (1977) Acoustic orientation. In: Wimsatt WA (ed) Biology of bats, vol III. Academic Press, New York London, pp 74–239
Oldfield BP (1980) Accuracy of orientation in female crickets,Teleogryllus oceanicus (Gryllidae): Dependence on song spectrum. J Comp Physiol 141:93–99
Paton JA (1975) Frequency analysis in the auditory system of field crickets. PhD Thesis, Cornell University, Ithaca, New York
Paton JA, Capranica RR, Dragsten PR, Webb WW (1977) Physical basis for auditory frequency analysis in field crickets (Gryllidae). J Comp Physiol 119:221–240
Pollack GS (1982) Sexual differences in cricket calling song recognition. J Comp Physiol 146:217–221
Pollack GS, Hoy RR (1979) Temporal patterns as a cue for species-specific calling song recognition in crickets. Science 204:429–432
Pollack GS, Hoy RR (1981) Phonotaxis in flying crickets: Neural correlates. J Insect Physiol 27:41–45
Pollack GS, Plourde N (1982) Directionality of acoustic orientation in flying crickets. J Comp Physiol 146:207–215
Pollack GS, Huber F, Weber T (1984) Frequency and temporal pattern dependent phonotaxis ofTeleogryllus oceanicus in flight and walking paradigms. J Comp Physiol A 154:13–26
Popov AV, Shuvalov VF (1977) Phonotactic behavior of crickets. J Comp Physiol 119:111–126
Popov AV, Shuvalov VF, Markovich AM (1975) Spectrum of the calling songs, phonotaxis and the auditory system in the cricketGryllus bimaculatus. J Evol Biochem Physiol 2:453–460
Ragge DH (1972) An unusual case of mass migration by flight inGryllus bimaculatus De Geer (Orthoptera Gryllidae). Bulletin de l'IFAN vol, 34 A, no. 4
Roeder KD, Treat AE (1957) Ultrasonic reception by the tympanal organ of noctuid moths. J Exp Zool 134:127–158
Roeder KD, Treat AE (1961) The detection and evasion of bats by moths. Am Sci 49:135–148
Roeder KD (1966) Acoustic sensitivity of the noctuid tympanic organ and its range for the cries of bats. J Insect Physiol 12:843:859
Roeder KD (1967a) Nerve cells and insect behavior. Harvard University Press, Cambridge Mass
Roeder KD (1967b) Turning tendency of moths exposed to ultrasound while in stationary flight. J Insect Physiol 13:873–888
Roeder KD (1972) Acoustic and mechanical sensitivity of the distal lobe of the pilifer in choerocampine hawkmoths. J Insect Physiol 18:1249–1264
Roeder KD, Treat AE, Vande Berg JS (1968) Auditory sense in certain sphingid moths. Science 159:331–333
Schaller F, Timm C (1950) Das Hörvermögen der Nachtschmetterlinge. Z Vergl Physiol 32:468–481
Schildberger K (1984) Temporal selectivity of identified auditory neurons in the cricket brain. J Comp Physiol A 155:171–185
Simons JA, Kick SA (1983) Interception of flying insects by bats. In: Huber F, Markl H (eds) Neuroethology and behavioral physiology. Springer, Berlin Heidelberg New York, pp 267–279
Smith AG (1976) Black field cricket (Teleogryllus commodus): Life cycle. Dept of scientific and industrial research, Wellington, New Zealand, Information series No 105/23
Thorson J, Weber T, Huber F (1982) Auditory behavior of the cricket II. Simplicity of calling song recognition inGryllus, and anomalous phonotaxis at abnormal carrier frequencies. J Comp Physiol 146:361–378
Tomioka K, Yamaguchi T (1980) Steering response of adult and nymphal crickets to light, with special reference to the head rolling movement. J Insect Physiol 26:47–57
Vaughan TA (1976) Nocturnal behavior of the African false vampire bat (Cardioderma cor). J Mammol 57:227–248
Walker TJ (1957) Specificity in the response of female tree crickets (Orthoptera, Gryllidae, Oceanthinae) to calling songs of the males. Ann Ent Soc Am 50:626–636
Weber T, Thorson J, Huber F (1981) Auditory behavior of the cricket. I. Dynamics of compensated walking and discrimination paradigms on the Kramer treadmill. J Comp Physiol 141:215–232
Whitaker JR IO, Black HL (1976) Food habits of bats from Zambia, Africa. J Mammol 57:199–204
Wine JJ, Krasne FB (1982) The cellular organization of crayfish escape behavior. In: Bliss DE (ed) The biology of Crustacea, vol 4, Sandeman DB, Atwood HL (eds) Neural integration and behavior. Academic Press, New York London, pp 242–292
Wohlers DW, Huber F (1982) Processing of sound signals by six types of neurons in the prothoracic ganglion of the cricket,Gryllus bimaculatus L. J Comp Physiol 146:161–173
Zaretsky MD (1972) Specificity of the calling song and short term changes in the phonotactic response by female crickets (Scapsipedus marginatus) (Gryllidae). J Comp Physiol 79:153–172
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nolen, T.G., Hoy, R.R. Phonotaxis in flying crickets. J. Comp. Physiol. 159, 423–439 (1986). https://doi.org/10.1007/BF00604163
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00604163