Abstract
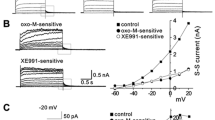
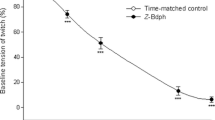
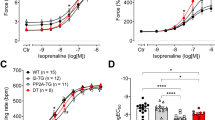
The mechanism of muscarinic inhibition of the Ca-current (I Ca) was studied in ventricular myocytes of guinea pig hearts and the following results were obtained. 1. Acetylcholine (ACh) in concentrations up to 10−4 M had little effect, if any, onI Ca in control cells. 2. ACh reduced the isoprenaline (ISP)-induced increase ofI Ca. The doseresponse-relation (ISP concentration vs.I Ca density) was shifted by ACh towards higher ISP concentrations. But both, at low and high ISP concentrations ACh had nor or little effect. 3. ACh was ineffective whenI Ca was increased by dialysing the cell with catalytic subunit of cAMP-dependent protein kinase or cAMP. 4. ACh reducedI Ca enhanced by isobutylmethylxanthine or by forskolin. 5. ACh did not depressI Ca when the cell was dialysed with the nonhydrolysable GTP-derivative, GMP-PNP. In this condition the β-adrenergic enhancement ofI Ca was also absent. 6. Pertussis toxin, which is known to inhibit the inhibitory transducerprotein (Ni), abolished the ACh response.
We concluded from these results that ACh depressesI Ca by inhibiting, via Ni, the cAMP production.
Similar content being viewed by others
References
Bailey J, Watanabe A, Besch H, Lathrop D (1979) Acetylcholine antagonism of the electrophysiological effects of isoproterenol on canine cardiac Purkinje fibers. Circ Res 44:378–382
Berridge M, Irvine R (1984) Inositol triphosphate, a novel second messenger in cellular signal transduction. Nature 312:315–321
Biegon R, Pappano A (1980) Dual mechanism for inhibition of calcium-dependent action potentials in avian ventricular muscle. Circ Res 46:353–362
Biegon R, Epstein P, Pappano A (1980) Muscarinic antagonism of effects of phosphodiesterase inhibitor (methylisobutylxanthine) in embryonic chick ventricle. J Pharmacol Exp Ther 215:348–356
Breitwieser G, Szabo G (1985) Uncoupling of cardiac muscarinic and β-adrenergic receptors from ion channels by guanine nucleotide analogue. Nature 317:538–540
Brown B, Polson J, Krzanowski J, Wiggins J (1980) Influence of isoproterenol and methylisobutylxanthine on the contractile and cyclic nucleotide effects of methacholine in isolated rat atria. J Pharmacol Exp Ther 212:325–332
Carmeliet E, Mubagwa K (1986) Changes by acetylcholine of membrane currents in rabbit cardiac Purkinje fibres. J Physiol 371:201–217
Codina J, Hildebrandt J, Sunyer T, Sekura R, Manclark C, Iyengar R, Birnbaumer L (1984) Mechanism in vectorial receptoradenylate cyclase signal transduction. Adv Cyclic Nucleotide Res 17:111–125
Daly J (1984) Forskolin, adenylate cyclase, and cell physiology: an overview. Adv Cyclic Nucleotide Res 17:81–89
Endoh M (1980) The time course of changes in cyclic nucleotide levels during cholinergic inhibition of positive inotropic actions of isoprenaline and theophylline in the isolated canine ventricular myocardium. Naunyn-Schmiedeberg's Arch Pharmacol 312:175–182
Flitney FW, Singh J (1980) Depressant effect of 8-bromo guanosine 3′,5′-cyclic monophosphate on endogenous adenosoine 3′–5′-cyclic monophosphate levels in frog ventricle. J Physiol (Lond) 302:29P-30P
Gilman A (1984) G proteins and dual control of adenylate cyclase. Cell 36:577–579
Hamill O, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Hazeki O, Ui M (1981) Modification by islet-activating protein of receptor-mediated regulation of cyclic AMP accumulation in isolated rat heart cells. J Biol Chem 256:2856–2862
Hescheler J, Kameyama M, Trautwein W (1985) Mechanism of inhibition of the calcium current (I Ca) by acetylcholine (ACh) in isolated ventricular cells of guinea pig heart. Pflügers Arch 405 (suppl):R26
Hino N, Ochi R (1980) Effects of acetylcholine on membrane currents in guinea-pig papillary muscle. J Physiol 307:185–197
Hoffman B, Suckling E (1953) Cardiac cellular potentials: effect of vagal stimulation and acetylcholine. Am J Physiol 173:312–320
Iijima T, Irisawa H, Kameyama M (1985) Membrane currents and their modification by acetylcholine in isolated single atrial cells of the guinea-pig. J Physiol 359:485–501
Inui J, Imamura H (1977) Effects of acetylcholine on calciumdependent electrical and mechanical responses in the guinea-pig papillary muscle partially depolarized by potassium. Naunyn-Schmiedeberg's Arch Pharmacol 299:1–7
Isenberg G, Klöckner U (1982) Calcium tolerant ventricular myocyctes prepared by preincubation in a “KB medium”. Pflügers Arch 395:6–18
Jakobs K, Aktories K, Schultz G (1979) GTP-dependent inhibition of cardiac adenylate cyclase by muscarinic cholinergic agonists. Naunyn-Schmiedeberg's Arch Pharmacol 310:113–119
Josephson I, Sperelakis N (1982) On the ionic mechanism underlying adrenergic-cholinergic antagonism in ventricular muscle. J Gen Physiol 79:69–86
Kameyama M, Hofmann F, Trautwein W (1985) On the mechanism of β-adrenergic regulation of the Ca channel in the guinea-pig hear. Pflügers Arch 405:285–293
Kameyama M, Hescheler J, Hofmann F, Trautwein W (1986) Modulation of Ca current during the phosphorylation cycle in guinea pig heart. Pflügers Arch 407:123–128
Keely S, Lincoln T, Corbin J (1978) Interaction of acetylcholine and epinephrine on heart cyclic AMP-dependent protein kinease. Am J Physiol 234(4):H432-H438
Krebs E, Beavo J (1979) Phosphorylation-dephosphorylation of enzymes. Ann Rev Biochem 48:923–959
Levitan I (1985) Phosphorylation of ion channels. J Mem Biol 87:177–190
Levy MN (1983) Neural control of cardiac rythm contraction. In: Rosen M, Hoffman B (eds) Cardiac therapy. Martinus Nijhoff Publishers, Boston, pp 73–94
Linden J, Brooker G (1979) The questionable role of cyclic guanosine 3′:5′-monophosphate in heart. Biochem Pharmacol 28:3351–3360
Linden J, Hollen C, Patel A (1985) The mechanism by which adenosine and cholinergic agents reduce contractility in rat myocardium: correlation with cyclic AMP and receptor densities. Circ Res 56:728–735
Löffelholz K, Pappano A (1985) The parasympathic neuroeffector junction of the heart. Pharmacol Rev 37:1–24
MacLeod KM (1985) The interaction of carbachol and Forskolin in rabbit papillary muscles. Eur J Pharmacol 107:95–99
Meester W, Hardman H (1967) Blockade of the positive inotropic actions of epinephrine and theophylline by acetylcholine. J Pharmacol Exp Ther 158:241–247
Metzger H, Lindner E (1981) The positive inotropic-acting forskolin, a potent adenylatecyclase activator. Drug Res 31:1248–1250
Nargeot J, Nerbonne J, Engels J, Lester H (1983) Time course of the increase in the myocardial slow inward current after photochemically generated concentration jump of intracellular cAMP. Proc Natl Acad Sci USA 80:2395–2399
Nawrath H (1977) Does cyclic GMP mediate the negative inotropic effect of acetylcholine in the heart? Nature 267:72–74
Nestler E, Greengard P (1983) Protein phosphorylation in the brain. Nature 305:583–588
Ochi R (1981) Decrease in calcium conductance by acetylcholine in mammalian ventricular muscle. In: Ohnishi T, Endo M (eds) The mechanism of gated calcium transport across biological membranes. Academic Press, New York, pp 79–86
Osterrieder W, Brum G, Hescheler J, Trautwein W, Flockerzi V, Hofmann F (1982) Injection of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature 298:576–578
Pappano AJ, Hartigan PM, Coutu MD (1982) Acetylcholine inhibits the positive inotropic effect of cholera toxin in ventricular muscle. Am J Physiol 243:H434-H441
Rodbell M (1980) The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature 284:17–22
Rodger IW, Shahid M (1984) Forskolin, cyclic nucleotides and positive inotropism in isolated papillary muscles of the rabbit. Br J Pharmac 81:151–159
Schmidt RF (1958) Über die Acetylcholin-Empfindlichkeit verschiedener Herzabschnitte. Naunyn-Schmiedeberg's Arch Pharmacol 233:531–540
Seamon K, Daly J (1983) Forskolin, cyclic AMP and cellular Physiology. Trends Pharmacol Sci 4:120–123
Seamon K, Wetzel B (1984) Interaction of forskolin with dually regulated adenylate cyclase. Adv Cyclic Nucleotide Res 17:91–99
Selinger Z, Cassel D (1981) Role of guanine nucleotides in hormonal activation of adenylate cyclase. Adv Cyclic Nucleotide Res 14:15–22
Shigenobu J, Sperelakis N (1972) Ca2+ current chanels induced by catecholamines in chick embryonic hearts whose fast Na+ channels are blocked by tetrodotoxin or elevated K+ Circ Res 31:932–952
Sulakhe PV, Phan NT, Jagadeesh G (1985) Comparison of cholinergic inhibition and beta-adrenergic stimulation of adenylate cyclase from rat and guinea-pig hearts: effects of guanine nucleotides and monovalent cations. Gen Pharmac 16:311–320
Trautwein W, Kameyama M (1986) The mechanism of β-adrenergic regulation of Ca-channels. In: Intracellular injection and patchclamp studies. In: Noble D, Powell T (eds) Electrophysiology of single cardiac cells. Academic Press, London, in press
Ui M (1984) Islet-activating protein, pertussis toxin: a probe for functions of the inhibitory guanine nucleotide regulatory component of adenylate cyclase. TIPS July 1984:277–279
Watanabe A, Besch H (1975) Interaction between cyclic adenosine monophosphate and cyclic guanosine monophosphate in guinea pig ventricular myocardium. Circ Res 37:309–317
Watanabe M, Lindemann P, Fleming J (1984) Mechanisms of muscarinic modulation of protein phosphorylation in intact ventricles. Fed Proc 43:2618–2623
West R, Moss J, Vaughan M, Liu T, Liu TY (1986) Pertussis toxin-catalyzed ADP-ribosylation of transducin. J Biol Chem 260:15718–15722
Wolff J, Hope Cook G, Goldhammer A, Londons C, Hewlett E (1984) Bordetella pertussis: multiple attacks on host cell cyclic AMP regulation. Adv Cyclic Nucleotide Res 17:161–172
Author information
Authors and Affiliations
Additional information
This work was supported by the Deutsche Forschungsgemeinschaft, SFB 38 (Membranforschung), Project G
Rights and permissions
About this article
Cite this article
Hescheler, J., Kameyama, M. & Trautwein, W. On the mechanism of muscarinic inhibition of the cardiac Ca current. Pflugers Arch. 407, 182–189 (1986). https://doi.org/10.1007/BF00580674
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00580674