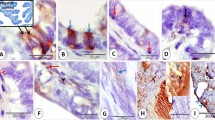
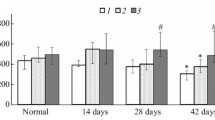
Summary
The synthesis of glycoproteins in rabbit uterine epithelium during the late preimplantation period was studied using tritiated N-acetylglucosamine. In vivo labelling was achieved by the intra-uterine implantation of agar gel columns containing the precursor. Autoradiography showed the radioactivity to be predominantly localized in the apical cell surfaces, with single cells exhibiting an accumulation of silver grains in their supranuclear cytoplasm. After gel electrophoresis of uterine flushings, activity was mainly found in the β-glycoprotein fraction. Fluorescein isothiocyanate (FITC)-conjugated wheat-germ agglutinin reacted with the apical cytoplasm and surfaces of the endometrial cells. However, FITC-conjugated concanavalin A exhibited a different binding pattern, reacting first with the basal cytoplasm, and later with the apical cytoplasm. After concanavalin-A staining, single cells exhibited positive vesicles in their lateral and apical parts. These cells may be released into the uterine lumen until 210 h post coitum. Neither of the lectins reacted with ciliated cells. Concanavalin A showed an affinity for the β-glycoprotein fraction of the uterine secretion. The results indicate that, although all endometrial cells contain glycoproteins, only a few of these seem to be involved in the synthesis of secretory products.
Similar content being viewed by others
References
Abe T, Endo M, Yosizawa Z (1972) Study on the uterine mucosubstances accompanying the infertility effect of an intrauterine device. Clin Chim Acta 42:29–35
Appleton TC (1966) Resolving power, sensitivity and latent imagefading of soluble-compound autoradiographs. J Histochem Cytochem 14:414–420
Baker JE (1982) Application of capillary thin layer isoelectric focusing in polyacrylamide gel to the study of alkaline proteinases in stored-product insects. Comp Biochem Physiol 71(B):501–506
Beier HM (1978) Physiology of uteroglobin. Reprod Physiol 8:219–248
Bennett G, Leblond CP (1970) Formation of cell coat material for the whole surface of columnar cells in the rat small intestine, as visualized by radioautography with l-fucose-3H. J Cell Biol 46:409–416
Bennett G, Leblond CP, Haddad A (1974) Migration of glycoprotein from the golgi apparatus to the surface of various cell types as shown by radioautography after labeled fucose injection into rats. J Cell Biol 60:258–284
Bennett G, Kan FWK, O'Shaughnessy D (1981) The site of incorporation of sialic acid residues into glycoproteins and the subsequent fates of these molecules in various rat and mouse cell types as shown by radioautography after injection of 3H-N-acetylmannosamine. II. Observations in tissues other than liver. J Cell Biol 88:16–28
Bochskanl R, Kirchner C (1981) Uteroglobin and the accumulation of progesterone in the uterine lumen of the rabbit. Wilhelm Roux's Arch 190:127–131
Bochskanl R, Thie M, Kirchner C (1984) Progesterone dependent uptake of uteroglobin by rabbit endometrium. Histochemistry 80:581–589
Bonner WM, Laskey RA (1974) A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem 46:83–88
Burger MM, Goldberg AR (1967) Identification of a tumor-specific determinant on neoplastic cell surfaces. Proc Natl Acad Sci USA 57:359–366
Chilton BS, Nicosia SV, Laufer MR (1980) Effects of estradiol-17β on endocervical cytodifferentiation and glycoprotein biosythesis in the ovariectomized rabbit. Biol Reprod 23:677–686
Cook GMW (1968) Glycoproteins in membranes. Biol Rev 43:363–391
Davis BJ (1964) Disc electrophoresis. II. Method and application to human serum proteins. Ann NY Acad Sci 121:404–427
Davis BW, Berger EG, Locher GW, Zeller M, Goldhirsch A (1984) Immunhistochemical localization of galactosyltransferase in the normal human ovary and fallopian tube. J Histochem Cytochem 32:92–96
Denker HW (1970) Topochemie hochmolekularer Kohlenhydratsubstanzen in Frühentwicklung und Implantation des Kaninchens. I. Allgemeine Lokalisierung und Charakterisierung hochmolekularer Kohlenhydratsubstanzen in frühen Embryonalstadien. Zool Jb Physiol 75:141–245
Dunbar BS, Daniel JC (1979) High molecular weight components of rabbit uterine fluids. Biol Reprod 21:723–733
Enders AC, Schlafke S, Welsh AO (1980) Trophoblastic and uterine luminal epithelial surfaces at the time of blastocyst adhesion in the rat. Am J Anat 159:59–72
Endo M, Yosizawa Z (1973) Hormonal effect on glycoproteins and glycosaminoglycans in rabbit uteri. Arch Biochem Biophys 156:397–403
Endo M, Yosizawa Z (1975a) Glycosaminoglycans and acidic glycoproteins in rabbit uterus under estrogenic conditions. Biochim Biophys Acta 404:274–280
Endo M, Yosizawa Z (1975b) Isolation and characterization of a sulfated glycoprotein from rabbit uterus. J Biochem 78:873–878
Endo M, Mori T, Yamasaki M, Yosizawa Z (1976) Histochemical localization of estrogen induced sulfated glycoproteins in rabbit uterus. Histochemistry 46:287–296
Etzler ME, Branstrator ML (1974) Differential localization of cell surface and secretory components in rat intestinal epithelium by use of lectins. J Cell Biol 62:329–343
Goldstein I, Hollerman JD, Smith EC (1965) Protein-carbohydrate interaction. II. Inhibition studies on the interaction of concanavalin A with polysaccharides. Biochemistry 4:876–883
Gonatas NK, Avrameas S (1973) Detection of plasma membrane carbohydrates with lectin peroxidase conjugates. J Cell Biol 59:436–443
Greenaway PJ, LeVine D (1973) Binding of N-acetyl-neuraminic acid by wheat germ agglutinin. Nature (New Biol) 241:191–192
Guillomot M, Flechon JE, Wintenberger-Torres S (1982) Cytochemical studies of uterine and trophoblastic surface coats during blastocys attachment in the ewe. J Reprod Fert 65:1–8
Joshi SG, Ebert KM (1976) Effects of progesterone on labelling of soluble proteins and glycoproteins in rabbit endometrium. Fertil Steril 27:730–739
Kirchner C (1969) Untersuchungen an uterussperzifischen Glykoproteinen während der frühen Gravidität des Kaninchens Oryctolagus cuniculus. Wilhelm Roux's Arch 164:97–113
Kirchner C (1971) Einfluß von Choriongonadotrophin auf die Sekretion eines uterusspezifischen Proteins des Kaninchens. Acta Endocrinol (Kbh) 68:394–400
Kirchner C (1980) Non-uteroglobin proteins in the rabbit. In: Beato M (ed) Steroid induced uterine proteins. Elsevier North-Holland Biomedical Press. Amsterdam, pp 69–86
Lee MC, Wu TC, Wan YJ, Damjanov I (1983) Pregnancy-related changes in the mouse oviduct and uterus revealed by differential binding of fluoresceinated lectins. Histochemistry 79:365–375
Lejeune B, Lecocq R, Lamy F, Leroy F (1982) Changes in the pattern of endometrial protein synthesis during decidulization in the rat. J Reprod Fert 66:519–523
Lis H, Sharon N (1973) The biochemistry of plant lectins (phytohemagglutinins). Annu Rev Biochem 42:541–574
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Luft JH (1961) Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol 9:409–414
Meiniel R, Meiniel A (1985) Analysis of the subcommissural organs of several vertebrate species by use of fluorescent lectins. Cell Tissue Res 239:359–364
Pestalozzi DM, Hess M, Berger EG (1982) Immunhistochemical evidence for cell surface and golgi localization of galactosyl-transferase in human stomach, jejunum, liver and pancreas. J Histochem Cytochem 30:1146–1152
Pohle W, Popov N, Schulzeck S, Matthies H (1982) Distribution of hippocampal glycoproteins as demonstrated in rats by lectin binding and autoradiography after intraventricular injections of labelled fucose, N-acetyl-glucosamine and mannose. neuroscience 11:2715–2724
Ricketts AP, Scott DW, Bullock DW (1984) Radioiodinated surface proteins of separated cell types from rabbit endometrium in relation to the time of implantation. Cell Tissue Res 236:421–429
Roberts GP, Parker JM (1974) An investigation of enzymes and hormone-binding proteins in the luminal fluid of the bovine uterus. J Reprod Fert 40:305–313
Salier JP, Faye L, Vergaine D, Martin JP (1980) True and false glycoprotein microheterogeneity observed with lectin crossed affinoimmunoelectrophoresis of interalpha-trypsin-inhibitor. Electrophoresis 1:193–197
Scheele GA (1975) Two-dimensional gel analysis of soluble proteins. Characterization of guinea pig exocrine pancreatic proteins. J Biol Chem 250:5375–5385
Thie M, Bochskanl R, Kirchner C (1984a) Purification and immunohistology of a glycoprotein secreted from the rabbit uterus before implantation. Cell Tissue Res 237:155–160
Thie M, Bochskanl R, Kirchner C (1984b) Zur Rolle des β-Glycoproteins im Implantationsgeschehen beim Kaninchen. In: Verhandlungsbericht der IX. Veterinär-Humanmedizinischen Gemeinschaftstagung “Physiologie und Patholggie der Fortpflanzung”, Hannover pp 139–143
Winzler RJ (1970) Carbohydrates in cell surfaces. Int Rev Cytol 29:77–114
Yeh KY, Moog F (1984) Biosynthesis and transport of glycoproteins in the small intestinal epithelium of rats. I. Developmental change and effect of hypophysectomy. Dev Biol 101:446–462
Young RW, Fulhorst HW (1965) Recovery of 35S radioactivity from protein-bearing polyacrylamide gel. Anal Biochem 11:389–391
Author information
Authors and Affiliations
Additional information
Supported by grants Ki 154/9-3 and 154/10-1 from the Deutsche Forschungsgemeinschaft
Rights and permissions
About this article
Cite this article
Thie, M., Bochskanl, R. & Kirchner, C. Glycoproteins in rabbit uterus during implantation. Histochemistry 84, 73–79 (1986). https://doi.org/10.1007/BF00493424
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00493424