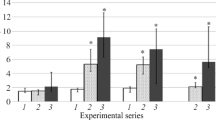
Experiments on female Chinchilla rabbits addressed the effects of progesterone on the functional activity of P-glycoprotein (Pgp). Animals were divided into four groups: animals of group 1 underwent sham operations; group 2 underwent ovariectomy; groups 3 and 4 underwent ovariectomy followed 15 days later by p.o. treatment with progesterone (2 and 15 mg/rabbit respectively). The functional activity of Pgp was determined seven days before the experiment started and on post-operative days 14, 28, and 42 by analysis of the pharmacokinetics of its marker substrate fexofenadine and serum sex hormone concentrations. At the end of the study, animals of each group were harvested by air embolism and specimens of large intestine were collected for immunohistochemical analysis. Ovariectomy led to a decrease in the functional activity of Pgp and a decrease in the staining intensity of the apical and lateral membranes of enterocytes, providing indirect evidence of a decrease in the expression of the transporter protein. Administration of progesterone at a dose of 2 mg/rabbit on the background of ovariectomy increased the functional activity of Pgp from the level seen in the ovariectomy group, though its activity remained decreased compared with the baseline level. This group showed no changes in the staining intensity of enterocyte membranes as compared with the ovariectomy group. Use of progesterone at a dose of 15 mg/rabbit after ovariectomy increased the functional activity of Pgp and the staining intensity of enterocyte apical and lateral membranes from the level seen in the ovariectomy group, restoring the level of transporter protein activity to that of intact animals, probably because of increased expression.




Similar content being viewed by others
References
V. G. Kukes, S. V. Grachev, D. A. Sychev, and G. V. Ramenskaya, Drug Metabolism. Scientific Basis of Personalized Medicine: Guidelines for Doctors [in Russian], GEOTAR- MEDia, Moscow (2008).
M. M. Gottesman, Annu. Rev. Med., 53, 615 – 627 (2002).
H. M. Braakman, M. J. Vaessen, P. A. Hofman, et al., Epilepsia, 52, 849 – 856 (2011).
W. Y. Kim and L. Z. Benet, Pharmac. Res., 21(7), 1284 – 1293 (2004).
M. Frohlich, N. Albermann, A. Sauer, et al., Biochem. Pharmacol., 68(12), 2409 – 2416 (2004).
M. C. Chang, Endocrinology, 79(5), 939 – 948 (1966).
I. Camacho-Arroyo, A. M. Pasapera, M. A. Cerbon, Neurosci. Lett., 214(1), 25 – 28 (1996).
M. V. Gatsanoga, I. V. Chernykh, A. V. Shchul’kin, and E. N. Yakusheva, Nauka Molodykh — Eruditio Juvenium, 3, 5 – 10 (2016).
H. Tahara, H. Kusuhara, E. Fuse, Y. Sugiyama, Drug Metabol. Disposit., 33(7), 963 – 968 (2005).
E. N. Yakusheva, I. V. Chernykh, A. V. Shchul’kin, and M. V. Gatsanoga, Ros. Med. Biol. Vestn. im. Akad. I. P. Pavlova, 3, 49 – 53 (2015).
N. N. Karkishchenko, V. V. Khoron’ko, S. A. Sergeeva, and V. N. Karkishchenko, Pharmacokinetics [in Russian], Feniks, Rostov-on-Don, (2001).
D. G. Fuhrich, B. A. Lessey, and R. F. Savaris, Anal. Quant. Cytopathol. Histpathol., 35(4), 210 – 216 (2013).
G. P. Vinson and I. Chester Jones, J. Endocrinol., 29, 185 – 191(1964).
US Food and Drug Administration, Center for Drug Evaluation and Research, Guidance for Industry: Drug Interaction Studies – Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm292362.pdf.
E. N. Yakusheva, I. V. Chernykh, A. V. Shchul’kin, et al., Ros. Fiziol. Zh. im. I. M. Sechenova, 100(8), 944 – 952 (2014).
V. Poongavanam, N. Haider, and G. F. Ecker, Bioorg. Med. Chem., 20(18), 5388 – 5395 (2012).
A. Geick, M. Eichelbaum, and O. Burk, J. Biol. Chem., 276, 14581 – 14587 (2001).
S. A. Kliewer, J. T. Moore, L. Wade, et al., Cell, 92(1), 73 – 82 (1998).
This study was supported by the Russian Foundation for Basic Research (Grant No. 16-04-00320a).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 7, pp. 3 – 8, July, 2018.
Rights and permissions
About this article
Cite this article
Shchul’kin, A.V., Chernykh, I.V., Yakusheva, E.N. et al. Experimental Studies of the Effects of Progesterone on the Functional Activity of P-Glycoprotein. Pharm Chem J 52, 587–592 (2018). https://doi.org/10.1007/s11094-018-1864-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1864-8