Abstract
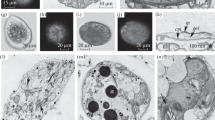
Three marine bacteria were examined for physiological and morphological changes in the initial phase of starvation. It was found that the starvation process was induced in a similar way irrespective of whether the cells were suspended in nutrient and energy free artificial seawater (NSS) or NSS supplemented with nitrogen and phosphorus. An initial phase of increased activity was consistent with a decreased response to added nutrients. Recovery from starvation exhibited the same response in both these starvation regimes, measured throughout the starvation period. Cells in nitrogen or phosphorus deprived starvation regimes, showed a high and rapid increased activity, followed by a delayed and more pronounced decline in respiratory activity. The initial phase of starvation also included a loss of poly-β-hydroybutyrate as observed by transmission electron microscopy (TEM). Two bacterial strains showed formation of small vesicles on the outer cell layer when examined by TEM. This formation and release of vesicles was related to the continuous size reduction during starvation survival. The results are discussed in terms of defining the mechanisms of initial cellular responses to nutrient deprivation.
Similar content being viewed by others
Abbreviations
- NSS:
-
nine salt solution
References
Amy PS, Morita RY (1983) Protein patterns of growing and starved cells of a marine Vibrio sp Appl Environ Microbiol 45:1748–1752
Baker RM, Singleton FL, Hood MA (1983) Effects of nutrient deprivation on Vibrio cholerae. Appl Environ Microbiol 46:930–940
Baxter M, Sieburth J McN (1984) Metabolic and ultrastructural response to glucose of two eurytrophic bacteria isolated from seawater at different enriching concentrations. Appl Environ Microbiol 47:31–38
Burleigh IG, Dawes EA (1967) Studies on the endogenous metabolism and senescence of starved Sarcina lutea. Biochem J 102:236–250
Chatterjee SN, Das J (1967) Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae J Gen Microbiol 49:1–11
Chatterjee SN, Adhikari P, Maiti M, Raychaudhuri C (1974) Growth of Vibrio cholerae cells: biochemical and electron microscopic study. Ind J Exp Biol 12:35–45
Dawes EA (1976) Endogenous metabolism and the survival of starved prokaryotes. In: Gray TRG, Postgate, IR (eds.) The survival of vegatative microbes. Cambridge University Press, Cambridge, pp 19–53
Dawson MP, Humphrey BA, Marshall KC (1981) Adhesion: a tactic in the survival strategy of a marine Vibrio during starvation. Curr Microbiol 6:195–199
Delieu T, Walker DA (1972) An improved cathode for the measurement of photosynthetic oxygen evolution by isolated chloroplasts New Phytol 71:201–205
Forberg CW, Beveridge T, Hellstrom A (1981) Cellulase and xylanase release from Bacteroides succinogenes and its importance in the rumen environment. Appl Environ Microbiol 42:886–896
Geesey GG, Morita RY (1981) Relationship of cell envelope stability to substrate capture in a marine psychophilic bacterium. Appl Environ Microbiol 42:553–540
Harder W, Dijkhuizen L (1983) Physiological responses to nutrient limitation. Ann Rev Microbiol 37:1–23
Hoekstra D, Laan JW van der, Leij L de, Whitholt B (1976) Release of outer membrane fragments from normally growing Escherichia coli. Biochim Biophys Acta 455:889–899
Humphrey B, Kjelleberg S, Marshall KC (1983) Responses of marine bacteria under starvation conditions at a solid-water interface. Appl Environ Microbiol 45:43–47
Jones KL, Rhodes-Roberts ME (1981) The survival of marine bacteria under starvation conditions. J Appl Bacteriol 50:247–258
Kjelleberg S, Humphrey BA, Marshall KC (1982) Effect of interfaces on small starved marine bacteria. Appl Environ Microbiol 43:1166–1172
Kjelleberg S, Humphrey BA, Marshall KC (1983) Initial phases of starvation and activity of bacteria at surfaces. Appl Environ Microbiol 46:978–984
Kjelleberg S, Hermansson M (1984) Starvation induced effects on bacterial surface characteristics. Appl Environ Microbiol 48:497–503
Matin A, Veldhuis C, Stegeman V, Veenhuis M (1979) Selective advantage of a Spirillum sp. in a carbon-limited environment. Accumulation of poly-β-hydroxybutyric acid and its role in starvation. J Gen Microbiol 112:349–355
Morita RY (1982) Starvation-survival of heterotrophs in the marine environment. In: Marshall KC (ed) Advances in microbial ecology, vol 6. Plenum Publ Corp, New York, pp 171–198
Novitsky JA, Morita RY (1976) Physiological characterization of small cells resulting from nutrient starvation of a psychrophilic marine Vibrio. Appl Environ Microbiol 32:617–622
Novitsky JA, Morita RY (1977) Survival of a psychrophilic marine Vibrio under long term nutrient starvation. Appl Environ Microbiol 33:635–641
Novitsky JA, Morita RY (1978) Possible strategy for the survival of marine bacteria under starvation conditions. Mar Biol 48:289–295
Poindexter JS (1981) Oligotrophy. Fast and famine existence. In: Alexander M (ed) Advances in microbial ecology, vol 5. Plenum Press, New York, pp 63–90
Reeve CA, Amy PS, Matin A (1984) Role of protein synthesis in the survival of carbon-starved Escherichia coli K-12. J Bacteriol 160:1041–1046
Reynolds ES (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J Cell Biol 17:208–212
Rothfield L, Pearlman-Kothencz M (1969) Synthesis and assembly of bacterial membrane components. A lipopolysacharidepospholipid protein complex excreted by living bacteria. J Mol Biol 44:477–492
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mårdén, P., Tunlid, A., Malmcrona-Friberg, K. et al. Physiological and morphological changes during short term starvation of marine bacterial islates. Arch. Microbiol. 142, 326–332 (1985). https://doi.org/10.1007/BF00491898
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00491898