Summary
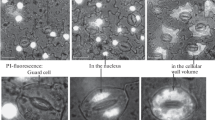
The effects of various chemical substances on the permeability of plasma membranes and tonoplasts of three suspension cultures (Catharanthus roseus, Thalictrum rugosum and Chenopodium rubrum) have been studied. The permeability of the plasma membrane is monitored by measuring the activity of the cytosolic enzyme isocitrate dehydrogenase and the permeability of the tonoplast is measured by determining the release of substances stored in the vacuoles (inorganic phosphate, berberine and betanin for the three cell lines, respectively). The minimum concentration required for quantitative release of vacuolar products have been established for five different permeabilization agents. Cell viability is lost upon permeabilization except for treatment of Catharanthus roseus with DMSO and Triton X-100.
Similar content being viewed by others
Abbreviations
- DMSO:
-
dimethylsulfoxide
- PEA:
-
phenethylalcohol
- HDTMAB:
-
hexadecyltrimethylammonium bromide
- ICDH:
-
isocitrate dehydrogenase
References
Brodelius P, Nilsson K (1983) Permeabilization of immobilized plant cells, resulting in release of intracellularly stored products with preserved cell viability. Eur J Appl Microbiol Biotechnol 17:275–280
Brodelius P, Vogel HJ (1985) A phosphorus-31 nuclear magnetic resonance study of phosphate uptake and storage in cultured Catharanthus roseus and Daucus carota plant cells. J Biol Chem 260:3556–3560
Brodelius P, Funk C, Shillito RD (1987) Permeabilization of cultivated plant cells by electroporation for release of intracellularly stored secondary products. Submitted
Brodelius P, Deus B, Mosbach K, Zenk MH (1979) Immobilized plant cells for the production and transformation of natural products. FEBS Lett 103:93–97
Felix H (1982) Permeabilized cells. Anal Biochem 120:211–234
Felix H, Brodelius P, Mosbach K (1981) Enzyme activities of the primary and secondary metabolism of simultaneously permeabilized and immobilized plant cells. Anal Biochem 116:462–470
Fuller KW, Bartlett DJ (1985) The chemosynthetic potential of plants and its realisation by immobilized systems. In: Fuller KW and Gallon JR (eds.) Plant products and the new Technology Oxford University Press, Oxford, UK, pp 229–247
Funk C, Gügler K, Brodelius P (1987) Increased secondary product formaton in plant cell suspension cultures after treatment with a yeast carbohydrate (elicitor). Phytochemistry 26:401–405
Linsmaier EM, Skoog F (1965) Organic growth factor requirements of tobacco tissue cultures. Physiol Plant 18:100–127
Lundberg P, Linsefors L, Vogel HJ, Brodelius P (1986) Permeabilization of plant cells: 31P NMR studies of the permeability of the tonoplast. Plant Cell Reports 5:13–16
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Neumann D, Krauss G, Hieke M, Gröger D (1983) Indole alkaloid formation and storage in cell suspension cultures of Catharanthus roseus. Planta Med 48:20–23
Parr AJ, Robins RJ, Rhodes MJC (1984) Permeabilization of Cinchona ledgeriana cells by dimethylsulfoxide. Effects on alkaloid release and long-term membrane integrity. Plant Cell Reports 3:262–265
Renaudin J-P, Guern J (1982) Compartmentation mechanisms of indole alkaloids in cell suspension cultures of Catharanthus roseus. Physiol Veg 20:533–547
Reuffer M (1985) The production of isoquinone alkaloids by plant cell cultures. In: Phillipson JD, Roberts NF and Zenk MH (eds.) The chemisty and biology of isoquinoline alkaloids. Springer-Verlag, Berlin Heidelberg, FRG, pp 265–280
Ushiyama K (1986) Mass production of plant tissue by tissue culture. Proceedings BIO FAIR TOKYO '86, October 15–18, Tokyo, Japan, pp 170–172
Wichers HJ (1985) Production of L-DOPA by suspension grown cells of Mucuna pruriens. PhD Thesis, Rijksuniversiteit te Groningen, Groningen, The Netherlands
Yamada Y, Fujita Y (1983) Production of Useful Compounds in Culture. In: Evans DA, Sharp WR, Ammirato PV and Yamada Y (eds.) Handbook of Plant Cell Culture, Vol. I, Macmillan Publishing Co, New York, pp 717–728
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brodelius, P. Permeabilization of plant cells for release of intracellularly stored products: viability studies. Appl Microbiol Biotechnol 27, 561–566 (1988). https://doi.org/10.1007/BF00451632
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00451632