Summary
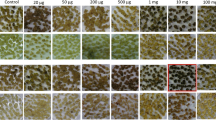
Lincomycin-resistant clones were isolated in diploid protoplast cultures of Nicotiana plumbaginifolia. Selection of the resistant clones was based on the ability of resistant calli to green in the presence of the antibiotic (1,000 mg l-1). Sensitive colonies formed white calli under the same conditions. In the absence of mutagenic treatment the frequency of the resistant clones was 1.0×10-4. This frequency could be increased up to 5.8×10-4 and 7.2×10-4 by treatment with 0.1 mM and 0.3 mM N-ethyl-N-nitrosourea (NEU), respectively.
Regenerated plants of 56 clones were tested for lincomycin resistance. Regenerates from all but seven clones were resistant to lincomycin, as demonstrated by leaf assay. The lincomycin-resistant regenerates tested were also resistant to clindamycin (a lincomycin derivative), but sensitive to streptomycin.
Regenerated plants in 17 clones were fully fertile and inherited lincomycin resistance maternally. Segregation for lincomycin resistance was observed in the seed progeny of five clones, which indicated maintenance of mixed cytoplasmic determinants after plant regeneration. Seed transmission of lincomycin resistance was confirmed in an additional 17 clones but the mode of inheritance (maternal or Mendelian) was not determined because of pollen sterility or reduced seed germination ability. These defects first appeared when the higher concentration of NEU was used. Various pigment deficiencies were also observed in a few clones.
Similar content being viewed by others
References
Bartlett SG, Harris EH, Grabowy TC, Gillham NW, Boyton IE (1979) Ribosomal subunits affected by antibiotic resistance mutations at seven chloroplast loci in Chlamydomonas reinhardtii. Mol Gen Genet 176: 199–208
Bayliss MW (1980) Chromosomal variation in plant tissues in culture. In: Vasil IK (ed) International review of cytology, Suppl 11A. Academic Press, New York, pp 113–144
Birky CW, Jr (1973) On the origin of mitochondrial mutants: evidence for intracellular selection of mitochondria in the origin of antibiotic resistant cells in yeast. Genetics 74: 421–432
Bourgin JP (1983) Protoplasts and the isolation of plant mutants. In: Potrykus I, Harms CT, Hinnen A, Hütter R, Shillito RD (eds) Protoplasts 1983. Lecture Proceedings. 6th International Protoplast Symposium. Birkhäuser Verlag, Basel, pp 43–50
Bourque DP, Horn NA, Capel MS (1977) Altered chloroplast ribosome of a streptomycin resistant mutant of Nicotiana tabacum. Plant Physiol Suppl 59: 110
Brettel RIS, Thomas E (1980) Reversion of Texas male-sterile cytoplasm maize in culture to give fertile, T-toxin resistant plants. Theor Appl Genet 58: 55–58
Cséplő Á, Maliga P (1982) Lincomycin resistance, a new type of maternally inherited mutation in Nicotiana plumbaginifolia. Curr Genet 6: 105–109
Cséplő Á, Nagy F, Maliga P Interspecific protoplast fusion to rescue a cytoplasmic lincomycin resistance mutation into fertile Nicotiana plumbaginifolia plants (submitted to Mol Gen Genet)
Fluhr R (1983) The segregation of organelles and cytoplasmic traits in higher plant somatic fusion hybrids. In: Potrykus I, Harms CT, Hinnen A, Hütter R, King PJ, Shillito RD (eds) Protoplasts 1983. Lecture Proceedings. 6th International Protoplast Symposium. Birkhäuser Verlag, Basel, pp 85–92
Fluhr R, Aviv D, Edelman M, Galun E (1983) Cybrids containing mixed and sorted-out chloroplasts following interspecific somatic fusions in Nicotiana. Theor Appl Genet 65: 289–294
Galun E (1982) Somatic cell fusion for inducing cytoplasmic exchange: a new biological system for cytoplasmic genetics in higher plants. In: Vasil IK, Frey KJ, Scowcroft WR (eds) Plant improvement and somatic cell genetics. Academic Press, New York, pp 205–219
Gengenbach BG, Green EC, Donovan CM (1977) Inheritance of selected pathotoxin resistance in maize plants regenerated from cell cultures. Proc Natl Acad Sci USA 74: 5113–5117
Hagemann R (1982) Induction of plastom mutations by nitrosourea-compounds. In: Edelman M, Hallick RB, Chua N-H (eds) Methods in chloroplast molecular biology. Elsevier Biomedical Press, Amsterdam, pp 119–127
Kao KW, Constabel F, Michayluk MR, Gamborg OL (1974) Plant protoplast fusion and growth of intergeneric hybrid cells. Planta 120: 215–227
Lee RW, Haughn GW (1980) Induction and segregation of chloroplast mutations in vegetative cell cultures of Chlamydomonas reinhardtii. Genetics 96: 79–94
Magerlein BJ, Birkenmayer RD, Kagan F (1967) Chemical modification of lincomycin. Antimicrob Agents Chemother 1966: 724–736
Maliga P (1984) Protoplasts in mutant selection and characterization. In: Giles KL (ed) International review of cytology, Suppl 16. Academic Press, New York, pp 161–167
Maliga P, Sz-Breznovits Á, Márton L (1973) Streptomycin resistant plants from callus culture of haploid tobacco. Nature New Biol 244: 28–30
Maliga P, Sz-Breznovits Á, Márton L (1975) Non-Mendelian streptomycin resistant tobacco mutant with altered chloroplasts and mitochondria. Nature (London) 255: 401–402
Maliga P, Sidorov VA, Cséplő Á, Menczel L (1981) Induced mutations in advancing in vitro culture techniques. In: International Atomic Energy Agency (eds) Induced mutations — a tool in plant research. IAEA, Austria, pp 339–352
Maliga P, Sidorov VA, Márton L, Cséplő Á, Medgyesy P, Trinh Manh Dung, Lázár G, Nagy F (1982a) Cell culture mutants and their uses. In: Vasil IK, Frey KJ, Scrowcroft WR (eds) Plant improvement and somatic cell genetics. Academic Press, New York, pp 221–237
Maliga P, Lörz H, Lázár G, Nagy F (1982b) Cytoplast-protoplast fusion for interspecific chloroplast transfer in Nicotiana. Mol Gen Genet 185: 211–215
Márton L, Dung TM, Mendel RR, Maliga P (1982) Nitrate reductase deficient cell lines from haploid protoplast cultures of Nicotiana plumbaginifolia. Mol Gen Genet 182: 301–304
Menczel L, Nagy F, Kiss Zs, Maliga P (1981) Streptomycin resistant and sensitive somatic hybrids of Nicotiana tabacum and Nicotiana knightiana: Correlation of resistance to Nicotiana tabacum plastids. Theor Appl Genet 59: 191–195
Menczel L, Galiba G, Nagy F, Maliga P (1982) Effect of radiation dosage on efficiency of chloroplast transfer by protoplast fusion in Nicotiana. Genetics 100: 487–495
Menczel L, Nagy F, Lázár G, Maliga P (1983) Transfer of cytoplasmic male sterility by selection for streptomycin resistance after protoplast fusion in Nicotiana. Mol Gen Genet 189: 365–369
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assay with tobacco tissue culture. Plant Physiol 15: 473–497
Nagy JI, Maliga P (1976) Callus induction and plant regeneration from mesophyll protoplasts of Nicotiana sylvestris. Z Pflanzenphysiol 78: 453–455
Nierhaus KH, Wittmann HG (1980) Ribosomal function and its inhibition by antibiotics in prokaryotes. Naturwissenschaften 67: 234–250
Sager R (1972) Cytoplasmic genes in Chlamydomonas. In: Cytoplasmic genes and organelles. Academic Press, New York, pp 49–104
Schlanger G, Sager R (1974) Localization of five antibiotic resistances at the subunit level in chloroplast ribosomes of Chlamydomonas. Proc Natl Acad Sci USA 71: 1715–1719
Umbeck PF, Gengenbach BG (1983) Reversion of male-sterile T-cytoplasm maize to male fertility in tissue culture. Crop Sci 23: 584–589
Umiel N (1979) Streptomycin resistance in tobacco III. A test on germinating seedlings indicates cytoplasmic inheritance in the St-R701 mutant. Z Pflanzenphysiol 93: 295–301
Yurina NP, Odintsova MS, Maliga P (1978) An altered chloroplast ribosomal protein in a streptomycin resistant mutant. Theor Appl Genet 52: 125–128
Author information
Authors and Affiliations
Additional information
Communicated by G. Melchers
Rights and permissions
About this article
Cite this article
Cséplő, Á., Maliga, P. Large scale isolation of maternally inherited lincomycin resistance mutations, in diploid Nicotiana plumbaginifolia protoplast cultures. Molec. Gen. Genet. 196, 407–412 (1984). https://doi.org/10.1007/BF00436187
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00436187