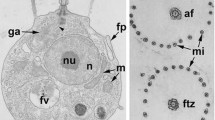
Abstract
The polar organelle of bacteria presumably is part of the flagellar apparatus. In order to characterize this structure, cytochemical studies on Sphaerotilus natans have been performed.
Marked ATPase activity is associated with the inner boundary layer and central layer of this organelle. The spaces between the boundary layers and the central layer of the polar organelle which are traversed by fine fibrilles are positive for reactions with diaminobenzidine. This indicates cytochrome oxidase activity.
S. natans possesses a ribbon-like, helically shaped polar organelle which is divided concomitantly with cell fission, possibly explaining inheritance of this structure and of the flagellar apparatus.
Similar content being viewed by others
References
Abram D (1965) Electron microscope observations in intact cells, protoplasts, and the cytoplasmic membrane of Bacillus stearothermophilus. J Bacteriol 89:855–873
Anderson WA (1970) The localization of cytochrome c oxidase activity during mitochondrial specialization in spermiogenesis of prosobranch snails. J Histochem Cytochem 18:201–210
Berg HC, Khan S (1983) A model for the flagellar rotary motor. In: Sund H, Veeger C (eds) Mobility and recognition in cell biology. Walter de Gruyter, Berlin New York, pp 485–497
Block SM, Berg HC (1984) Successive incorporation of forcegenerating units in the bacterial rotary motor. Nature 309:470–472
Brodie AF, Gutnick DL (1972) Electron transport and oxidative phosphorylation in microbial systems. In: King TE, Klingenberg M (eds) Electron and coupled energy transfer in biological systems, vol 1B. Marcel Dekker, New York, pp 599–681
Cohen-Bazire G, London J (1967) Basal organelles of bacterial flagella. J Bacteriol 94:458–565
Coulton JW, Murray RGE (1978) Cell envelop associations of Aquaspirillum serpens flagella. J Bacteriol 136:1037–1049
Dulbecco R, Vogt M (1954) Plaque formation and isolation of pure lines with Poliomyolitis viruses. J exp Med 99:167–182
Eisenbach M, Adler J (1981) Bacterial cell envelops with functional flagella. J Biol Chem 256:8807–8814
Hayat MA (1972) Basic electron microscopy techniques. Van Nostrand Reinhold Co., New York Cincinnati Toronto London Melbourne
Hickman DD, Frenkel AW (1965) Observations on the structure of Rhodospirillum rubrum. J Cell Biol 25/Mitosis: 279–291
Hinkle PC, McCarty, RE (1978) How cells make ATP. Sci Amer 238:104–123
Hoeniger JFM, Tauschel H-D, Stokes JL (1973) The fine structure of Sphaerotilus natans. Can J Microbiol 19:309–313
Keeler RF, Ritchie AE, Bryner JH, Elmore J (1966) The preparation and characterization of cell walls and the preparation of flagella of Vibrio fetus. J Gen Microbiol 43:439–454
Kushnarev VM, Smirnova TA, Dudenkova LG (1970) Electron microscopy of adenosine triphosphatase in Escherichia coli cells. Can J Microbiol 16:449–453
Macnab RM (1983) Bacterial mobility: energization and switching of the flagellar motor. In: Sund H, Veeger C (eds) Mobility and recognition in cell biology. Walter de Gruyter, Berlin New York, pp 499–516
Macnab RM (1984) The bacterial flagellar motor. TIBS 9:185–188
Mitchell P (1984) Bacterial flagellar motors and osmoelectric molecular rotation by an axially transmembrane well and turnstile mechanism. FEBS Letters 176:287–294
Muñoz E (1982) Polymorphism and conformational dynamics of F1-ATPase from bacterial membranes, a model for the regulation of these enzymes on the basis of molecular plasticity. Biochim Biophys Acta 650:233–265
Muñoz E, Freer JH, Ellar DJ, Salton MRJ (1968) Membrane-associated ATPase activity from Micrococcus lysodeikticus. Biochim Biophys Acta 150:531–533
Muñoz E, Salton MRJ, Ng MH, Schor MT (1969) Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the “soluble” enzyme and properties of the membrane-bound enzyme. Eur J Biochem 7:490–501
Murray RGE, Birch-Andersen A (1963) Specialized structure in the region of the flagella tuft in Spirillum serpens. Can J Microbiol 9:393–401
Remsen CC, Watson CW, Waterbury JB, Trüper HG (1968) Fine structure of Ectothiorhodospira mobilis Pelsh. J Bacteriol 95:2374–2392
Reynolds ES (1963) The use of lead citrate at high pH as an electronopaque stain in electron microscopy. J Cell Biol 17:208–212
Ritchie AE, Keeler RF, Bryner JH (1966) Anatomical features of Vibrio fetus: electron microscopic survey. J Gen Microbiol 43:427–438
Ryter A, Kellenberger E, Birch-Andersen A, Maaløe O (1958) Etude au microscope désoxyribonucléique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch 13b:597–605
Seligman AM, Plapinger RE, Wasserkrug HL, Deb C, Hanker JS (1967) Ultrastructural demonstration of cytochrome oxidase activity by the Nadi reaction with osmiophilic reagents. J Cell Biol 34:787–800
Seligman AM, Karnovsky MJ, Wasserkrug HL, Hanker JS (1968) Nondroplet ultrastructural demonstration of cytochrome oxidase activity with a polymerizing osmiophilic reagent, diaminobenzidine (DAB). J Cell Biol 38:1–14
Tauschel H-D, Drews G (1969a) Der Geißelapparat von Rhodopseudomonas palustris. I. Untersuchungen zur Feinstruktur des Polorganells. Arch Mikrobiol 66:166–179
Tauschel H-D, Drews G (1969b) Der Geißelapparat von Rhodopseudomonas palustris. II. Entstehung und Feinstruktur der Geißel-Basalkörper. Arch Mikrobiol 66:180–194
Vaituzis Z (1973) Localization of adenosine triphosphate activity in motile bacteria. Can J Microbiol 19:1265–1267
Vaituzis Z, Doetsch RN (1969) Relationship between cell wall, cytoplasmic membrane, and bacterial motility. J Bacteriol 100:512–521
Wachstein M, Meisel E (1957) Histochemistry of hepatic phosphatase at a physiological pH. Am J clin Pathol 27:13–23
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. G. Drews on occasion of his 60th birthday
Rights and permissions
About this article
Cite this article
Tauschel, HD. ATPase and cytochrome oxidase activities at the polar organelle in swarm cells of Sphaerotilus natans: an ultrastructural study. Arch. Microbiol. 141, 303–308 (1985). https://doi.org/10.1007/BF00428841
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00428841