Summary
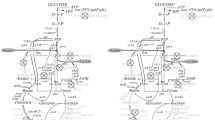
Treponema denticola was grown in serum-containing media to which 14C-labelled compounds were added. Determinations of radioactivity in the products formed indicated that the organism fermented alanine, cysteine, glycine, serine, and glucose. Fermentation products included acetate, lactate, succinate, formate, pyruvate, ethanol, CO2, H2S, and NH3. The products formed from glucose constituted a small portion of the total products. Assays of enzymatic activities in cell extracts indicated that the organism degraded glucose via the Embden-Meyerhof pathway. T. denticola possessed a coenzyme A-dependent CO2-pyruvate exchange activity associated with a clostridial-type clastic system for pyruvate metabolism. Phosphotransacetylase and acetate kinase activities were present in cell extracts. Acetyl phosphate formation and benzyl viologen reduction were detected when cell extracts were incubated with pyruvate, serine or cysteine. The data indicate that T. denticola is an amino acid fermenter and that it possesses the enzymes needed for the fermentation of glucose. However, glucose does not serve as the primary substrate when the organism grows in media including both this carbohydrate and amino acids.
Similar content being viewed by others
References
Ajello, Francesca: Activation of acetate in the treponemes (pathogenic Pallidum and non pathogenic Reiter and Kazan treponemes). G. Microbiol. 17, 107–114 (1969).
Barker, S. B., Summerson, W. H.: The colorimetric determination of lactic acid in biological material. J. biol. Chem. 138, 535–554 (1941).
Breznak, J. A., Canale-Parola, E.: Spirochaeta aurantia, a pigmented, facultatively anaerobic spirochete. J. Bact. 97, 386–395 (1969).
Burnett, G. W., Scherp, H. W.: Oral microbiology and infectious disease, 3rd ed. Baltimore: The Williams and Wilkins Co. 1968.
Canale-Parola, E., Holt, S. C., Udris, Z.: Isolation of free-living, anaerobic spirochetes. Arch. Mikrobiol. 59, 41–48 (1967).
—, Udris, Z., Mandel, M.: The classification of free-living spirochetes. Arch. Mikrobiol. 63, 385–397 (1968).
Feigl, F.: Spot tests in inorganic analysis, 5th ed. New York: Elsevier Publishing Co. 1958.
Feigl, F.: Spot tests in organic analysis, 6th ed. New York: Elsevier Publishing Co. 1960.
Fogo, J. K., Popowsky, M.: Spectrophotometric determination of hydrogen sulfide. Anal. Chem. 21, 732–734 (1949).
Friedemann, T. E., Haugen, G. E.: Pyruvic acid. II. The determination of keto acids in blood and urine. J. biol. Chem. 147, 415–442 (1943).
Hespell, R. B., Canale-Parola, E.: Carbohydrate metabolism in Spirochaeta stenostrepta. J. Bact. 103, 216–226 (1970a).
——: Spirochaeta litoralis sp. n., a strictly anaerobic marine spirochete. Arch. Mikrobiol. 74, 1–18 (1970b).
—, Joseph, R., Mortlock, R. P.: Requirement for coenzyme A in the phosphoroclastic reaction of anaerobic bacteria. J. Bact. 100, 1328–1334 (1969).
Inouye, M., Pardee, A. B.: Requirement of polyamines for bacterial division. J. Bact. 101, 770–776 (1970).
Kennedy, E. P., Barker, H. A.: Paper chromatography of volatile acids. Analyt. Chem. 23, 1033 (1951).
Lipmann, F., Tuttle, L. C.: A specific micromethod for the determination of acyl phosphates. J. biol. Chem. 159, 21–28 (1945).
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with the Folin phenol reagent. J. biol. Chem. 193, 265–275 (1951).
McCormick, N. G., Ordal, E. J., Whiteley, H. R.: Degradation of pyruvate by Micrococcus lactilyticus. I. General properties of the formate-exchange reaction. J. Bact. 83, 887–898 (1962).
Mortlock, R. P., Valentine, R. C., Wolfe, R. S.: Carbon dioxide activation in the pyruvate clastic system of Clostridium butyricum. J. biol. Chem. 234, 1653–1656 (1959).
Neish, A. C.: Analytical methods for bacterial fermentations. Nat. Res. Council of Canada, Report No. 46-8-3 (2nd revision), Saskatoon 1952.
Peck, H. D., Jr., Gest, H.: A new procedure for assay of bacterial hydrogenases. J. Bact. 71, 70–80 (1956).
Rose, I. A., Grunberg-Manago, M., Korey, S. R., Ochoa, S.: Enzymatic phosphorylation of acetate. J. biol. Chem. 211, 737–756 (1954).
Socransky, S. S.: Relationship of bacteria to the etiology of periodontal disease. J. dent. Res. 49, 203–222 (1970).
—, Listgarten, M., Hubersak, C., Cotmore, J., Clark, A.: Morphological and biochemical differentiation of three types of small oral spirochetes. J. Bact. 98, 878–882 (1969).
Stadtman, E. R., Novelli, G. D., Lipmann, F.: Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J. biol. Chem. 191, 365–376 (1951).
Stickland, L. H.: Studies in the metabolism of the strict anaerobes (genus Clostridium). I. The chemical reactions by which Cl. sporogenes obtains its energy. Biochem. J. 28, 1746–1759 (1934).
— Studies in the metabolism of the strict anaerobes (genus Clostridium). III. The oxidation of alanine by Cl. sporogenes. IV. The reduction of glycine by Cl. sporogenes. Biochem. J. 29, 889–898 (1935).
Strecker, H. J.: Formate fixation in pyruvate by Escherichia coli. J. biol. Chem. 189, 815–830 (1951).
Swim, H. E., Utter, M. F.: Isotopic experimentation with intermediates of the tricarboxylic acid cycle. In: S. P. Colowick and N. O. Kaplan (edit.) Methods in enzymology, vol. 4, pp. 584–609. New York: Academic Press Inc. 1957.
Umbreit, W. W., Burris, R. H., Stauffer, J. F.: Manometric techniques, 4th ed. Minneapolis, Minn.: Burgess Publishing Co. 1964.
Warburg, O., Christian, W.: Isolierung und Kristallisation des Gärungsferments Enolase. Biochem. Z. 310, 384–421 (1942).
Westerfeld, W. W.: A colorimetric determination of blood acetoin. J. biol. Chem. 161, 495–502 (1945).
Wolfe, R. S., O'Kane, D. J.: Cofactors of the phosphoroclastic reaction of Clostridium butyricum. J. biol. Chem. 205, 755–765 (1953).
——: Cofactors of the carbon dioxide exchange reaction of Clostridium butyricum. J. biol. Chem. 215, 637–643 (1955).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hespell, R.B., Canale-Parola, E. Amino acid and glucose fermentation by Treponema denticola . Archiv. Mikrobiol. 78, 234–251 (1971). https://doi.org/10.1007/BF00424897
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00424897