Summary
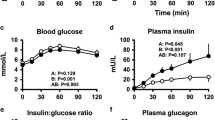
The secretion of pancreatic and gastrointestinal hormones in the basal state and after nutrient stimuli (50 g glucose, 50 g protein, or 30 g triglyceride administered on separate occasions) was assessed in ten previously type-1-diabetic patients after successful combined kidney and pancreas transplantation (systemic venous drainage). Fasting values were compared to matched non-diabetic kidney-transplanted patients and related to kidney function (endogenous creatinine clearance) and to the type and dosage of immunosuppressive medication. In the fasting state, only IR insulin concentrations were higher in pancreas-kidney-transplanted patients (by 88%; P=0.001) than in the kidney graft recipients. There were significant inverse correlations of plasma C-peptide, GIP, and gastrin immunoreactivity to endogenous creatinine clearance (kidney function). In response to nutrients, insulin secretion (IR insulin, C-peptide) was significantly stimulated by glucose, and — to a lesser degree — also by protein. Pancreatic glucagon was suppressed by glucose and stimulated by protein ingestion. GIP was raised after glucose and triglyceride more than after protein (P=0.0003). GLP-1 immunoreactivity was stimulated by all nutrients, with a tendency towards higher responses to protein and fat (P=0.06). Gastrin was mainly raised by protein. In conclusion, the overall pattern of pancreatic and gastrointestinal hormone release is normal in patients after combined pancreas-kidney-transplantation, but there are some peculiarities due to (a) systemic venous drainage of the pancreas graft (elevated fasting IR insulin) and (b) impaired kidney function (negative correlation of fasting plasma values to endogenous creatinine clearance for C-peptide, GIP, and gastrin). The plasma levels of these important regulatory peptides and their responses to nutrient stimulation are compatible with and may contribute to the well-preserved endocrine function of the pancreatic grafts (normal or slightly impaired glucose tolerance, preserved incretin effect).
Similar content being viewed by others
Abbreviations
- GIP:
-
Gastric inhibitory polypeptide (glucosedependent insulinotropic polypeptide)
- GLP-1:
-
Glucagon-like peptide 1 [7–36 amide] (proglucagon sequence 78–108 amide)
- IR:
-
immunoreactive
- WHO:
-
World Health Organization
References
Beard JC, Halter JB, Best JD, Pfeiffer MA, Porte D Jr (1984) Dexamethasone-induced insulin resistance enhances B cell responsiveness to glucose level in normal men. Am J Physiol 247:E592–596
Bilbrey GL, Faloona GR, White MG, Knochel JP (1974) Hyperglucagonemia of renal failure. J Clin Invest 53:841–847
Both GB, Dimitriadis GD, Pehling GB, et al (1984) Abnormal glucose counterregulation after subcutaneous insulin in insulin-dependent diabetes mellitus. N Engl J Med 310:1706–1711
Busing M, Nauck M, Hölzer H, et al (1990) Stimulation des transplantierten (denervierten) exokrinen Pankreas beim Menschen. Z Gastroenterol 25:468 (abstract)
Clark JDA, Wheatley T, Brons IGM, Bloom SR, Calne RY (1989) Studies of the entero-insular axis following pancreas transplantation in man: neural or hormonal control? Diabetic Med 6:813–817
Cleator IGM, Gourlay RH (1975) Release of immunoreactive gastric inhibitory polypeptide (IR-GIP) by oral ingestion of food substances. Am J Surg 130:128–135
Creutzfeldt W (1979) The incretin concept today. Diabetologia 16:75–85
Creutzfeldt W, Ebert R (1985) New developments in the incretin concept. Diabetologia 28:565–573
D'Allessandro AM, Stratta RJ, Sollinger HW, Kalayoglu M, Frisch JD, Belzer FO (1989) Use of UW solution in pancreas transplantation. Diabetes 38 [Suppl 1]:7–9
DeFronzo RA, Tobin JD, Rowe JW, Andres R. Glucose intolerance in uremia (1978) Quantification of pancreatic beta cell sensitivity to glucose and tissue sensitivity to insulin. J Clin Invest 62:425–435
Diem P, Abid M, Sutherland DER, Robertson RP (1989) Hyperglucagonemia and improved glucagon response to hypoglycemia in type I diabetic recipients of pancreas allografts. Diabetes 3 [Suppl]:48 A (abstract)
Diem P, Abid M, Redmon JB, Sutherland DER, Robertson RP (1990) Systemic venous drainage of pancreas allografts as independent cause of hyperinsulinemia in type 1 diabetic recipients. Diabetes 39:534–540
Ferrannini E, Cobelli C (1987) The kinetics of insulin in man. 11. The role of the liver. Diabetes Metab Rev 3:365–397
Fritsch W-P, Hausamen T-U, Rick W (1976) Gastric and extragastric gastrin in normal subjects and duodenal ulcer patients, and in patients with partial gastrectomy (Billroth I). Gastroenterology 71:552–557
Haffner J, Linnestad P, Schrumpf E, Hanssen LE, Flaten O, Oyasaeter S (1987) The immediate effect of human renal transplantation on basal and meal-stimulated levels of gastrointestinal hormones. Scand J Gastroenterol 22:42–46
Henriksen JH, Tronier B, Bülow JB (1987) Kinetics of circulating endogenous insulin, C-peptide, and proinsulin in fasting nondiabetic man. Metabolism 36:463–468
Ito C, Mito K, Hara H (1979) Review of the criteria for diagnosis of diabetes mellitus based on results of a followup-study. Diabetes 28:1039–1057
Kanatsuka A, Osegawa M, An T, Suzuki T, Hashimoto N, Makino H (1984) Augmented gastrin responses in diabetic patients with vagal neuropathy. Diabetologia 26:449–452
Kennedy FP, Bolli GB, Go VLW, Cryer PE, Gerich JE (1987) The significance of impaired pancreatic polypeptide and epinephrine responses to hypoglycemia in patients with insulin-dependent diabetes mellitus. J Clin Endokrinol Metab 64:602–608
Korman MG, Laver MC, Hansky J (1972) Hypergastrinaemia in chronic renal failure. Br Med J 1:209–210
Krarup T, Schwartz T, Hilsted J, Madsbad S, Verlaege O, Sestoft L (1979) Impaired response of pancreatic polypeptide to hypoglycaemia: an early sign of autonomic neuropathy in diabetics. Br Med J 2:1544–1546
Krarup T (1988) Immunoreactive gastric inhibitory polypeptide. Endocrine Rev 9:122–134
Krarup T, Nürnberg D, Mikkelsen K, et al (1990) How arterialized is blood sampled from a heated superficial hand vein? Diabetes 39 [Suppl 1]:88A (abstract)
Kreymann B, Ghatei MA, Williams G, Bloom SR (1987) Glucagon-like peptide 17–36: a physiological incretin in man. Lancet 11:1300–1304
Kuku SF, Japan JB, Emmanouel DS, Zeidler A, Katz AI, Rubenstein AH (1976) Heterogeneity of plasma glucagon. Circulating components in normal subjects and patients with chronic renal failure. J Clin Invest 58:742–750
Kuzio M, Dryburgh JR, Malloy KM, Brown JC (1974) Radioimmunoassay for gastric inhibitory polypeptide. Gastroenterology 66:357–364
Landgraf R, Nusser J, Müller W, et al (1989) Fate of late complications in type 1 diabetic patients after successful pancreas-kidney transplantation. Diabetes 38 [Suppl]:33–37
Lauritzen JB, Lauritzen KB, Ege Olson M, Timmerman I (1982) Gastric inhibitory polypeptide (GIP) and insulin release in response to oral and intravenous glucose in uremic patients. Metabolism 31:1096–1099
Luzi L, Secchi A, Facchini F, et al (1990) Reduction of insulin resistance by combined kidney-pancreas-transplantation in type 1 (insulin-dependent) diabetic patients. Diabetologia 33:549–556
Mayer G, Arnold R, Feurle G, Fuchs K, Ketterer H, Track NS, Creutzfeldt (1974) Influence of feeding and sham feeding upon serum gastrin and gastric acid secretion in control subjects and duodenal ulcer patients. Scand J Gastroenterol 9:703–710
Morel P, Balakumar M, Stevens B, Kendall D, Sutherland D (1990) Long term metabolic function of pancreas transplants and comparison between endocrine and exocrine function. Diabetes 39 [Suppl 1]: 160 A (abstract)
Müller WA, Faloona GR, Aguilar-Parada E, Unger RE (1970) Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 283:109–115
Mulholland MW, Debas HT (1988) Physiology and pathophysiology of gastrin: a review. Surgery 103:135–147
Nauck MA, Büsing M, Orskov C et al (1990) Preserved incretin effect after heterotopic pancreas transplantation in type 1-diabetic patients. Diabetologia 32:A 38 (abstract)
Nauck MA, Busing M, Orskov C, et al (1991) Sekretion von gastrointestinalen und Pankreas-Hormonen in Typ 1-diabetischen Patienten nach erfolgreicher kombinierter Pankreas- und Nierentransplantation. Akt Endokrinol Stoffw 12:111 (abstract)
Nerup J, Cathelineau C, Seignalet J, et al (1977) HLA and endocrine disease. In: Dausset J, Svejgaard A (eds) HLA and disease. Munksgaard, Copenhagen, pp 149–167
Ørskov C, Holst JJ (1987) Radio-immunoassays for glucagon-like peptides 1 and 2 (GLP-1 and GLP-2). Scand J Clin Lab Invest 47:165–174
Ørskov C, Jeppesen J, Madsbad S, Holst JJ (1991) Proglucagon products in plasma of noninsulin-dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest 87:415–423
Östman J, Bolinder J, Gunnarson R, et al (1989) Effects of pancreas transplantation on metabolic and hormonal profiles in IDDM patients. Diabetes 38 [Suppl 1]:88–93
O'Dorisio TM, Sirinek KR, Mazzaferri EL, Cataland S (1977) Renal effects on serum gastric inhibitory polypeptide (GIP). Metabolism 26:651–656
Osei K, Henry ML, O'Dorisio TM, Tesi RJ, Sommer BG, Ferguson RM (1990) Physiological and pharmacological stimulation of pancreatic islet hormone secretion in type 1 diabetic pancreas allograft recipients. Diabetes 39:1235–1342
Owyang C, Miller LJ, DiMagno EP, Brennan LA, Go VLW (1979) Gastrointestinal hormone profile in renal insufficiency. Mayo Clin Proc 54:769–773
Pagano G, Cavallo-Perin P, Cassader M, et al (1983) An in vivo and in vitro study of the mechanism of prednisoneinduced insulin resistance in healthy subjects. J Clin Invest 72:1814–1820
Paronen 1, Ala-Kaila K, Rantala I, Kainulainen H, Karvonen A-L (1991) Gastric parietal, chief, and G-cell densities in chronic renal failure. Scand J Gastroenterol 26:696–700
Pozza G, Bosi E, Secchi A, et al (1985) Metabolic control in type 1-(insulin-dependent) diabetes after pancreas transplantation. Br Med J 291:510–513
Regeur L, Faber OK, Binder C (1978) Plasma C-peptide in uraemic patients. Scand J Clin Lab Invest 38:771–775
Siegel EG, Creutzfeldt W (1989) Pancreatic transplantation or intensive insulin Baillère's Clin Gastroenterol 3:877–886
Somogyi M (1948) Studies of arteriovenous differences in blood sugar. I. Effect of alimentary hyperglycaemia on the rate of extrahepatic glucose assimilation. J Biol Chem 174:189–200
Taylor IL, Sells RA, McConnell RB, Dockray GJ (1980) Serum gastrin in patients with chronic renal failure. Gut 21:1062–1067
Theodorsson-Norheim E (1987) Friedman and Quade tests: basic computer program to perform nonoparametric twoway analysis of variance and multiple comparisons on ranks of several related samples. Comp Biol Med 17:85–99
Thompson DG, Wingate DL, Thomas M, Harrison D (1982) Gastric emptying as a determining of the oral glucose tolerance test. Gastroenterology 82:51–55
Tyden G, Brattström C, Bolinder J, et al (1989) Long-term metabolic control in recipients of segmental pancreas grafts with pancreaticoenterostomy or duct obstruction. Diabetes 38 [Suppl 1]:94–96
Unger RH (1985) Glucagon physiology and pathophysiology in the light of new advances. Diabetologia 28:574–578
Unger RH, Eisentraut AH (1969) Entero-insular axis. Arch Intern Med 123:261–266
Yovos JG, O'Dorisio TM, Pappas TN et al (1982) Effect of amino acids and gastric inhibitory polypeptide on insulin release in dogs. Am J Physiol 242:E53–58
Zukowska-Szczechowska, Grzeszczak W, Kokot F, Nieszporek T, Kusmierski S, Szkodny A (1987) Influence of type of immunosuppressive therapy on gastrin, insulin, glucagon, and pancreatic polypeptide secretion in kidney transplant patients. Transplant Proc 19:3731
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nauck, M.A., Büsing, M., Ørskov, C. et al. Basal and nutrient-stimulated pancreatic and gastrointestinal hormone concentrations in type-l-diabetic patients after successful combined pancreas and kidney transplantation. Clin Investig 70, 40–48 (1992). https://doi.org/10.1007/BF00422937
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00422937