Abstract
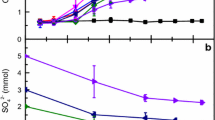
n-Hexadecane added as electron donor and carbon source to an anaerobic enrichment culture from an oil production plant or to anoxic marine sediment samples allowed dissimilatory sulfate reduction to sulfide. The enrichment from the oil field was purified via serial dilutions in liquid medium under a hexadecane phase and in agar medium with caprylate. A pure culture of a sulfate-reducing bacterium, strain Hxd3, with relatively tiny cells (0.4–0.5 by 0.8–2 μm) was isolated that grew anaerobically on hexadecane without addition of further organic substrates. Most of the cells were found to adhere to the hydrocarbon phase. It was verified that neither organic impurities in hexadecane nor residual oxygen were responsible for growth. Strain Hxd3 was grown with n-hexadecane of high purity (≥99.5%) in anoxic glass ampoules sealed by fusion. Of 0.4 ml hexadecane added per l (1.4 mmol per l), 90% was degraded with concomitant reduction of sulfate. Controls with pasteurized cells or a common Desulfovibrio species neither consumed hexadecane nor reduced sulfate. Incubation of cell-free medium with low reducing capacity and a redox indicator showed that the ampoules were completely oxygen-tight. Measured degradation balances and enzyme activities suggested a complete oxidation of the alkane to CO2 via the carbon monoxide dehydrogenase pathway. However, the first step in anaerobic alkane oxidation is unknown. On hexadecane, strain Hxd3 produced as much as 15 to 20 mM H2S, but growth was rather slow; with 5% inoculum, cultures were fully grown after 5 to 7 weeks. The new sulfate reducer grew on alkanes from C12 to C20, 1-hexadecene, 1-hexadecanol, 2-hexadecanol, palmitate and stearate. Best growth occurred on stearate (doubling time around 26 h). Growth on soluble fatty acids such as caprylate was very poor. Alkanes with chains shorter than C12, lactate, ethanol or H2 were not used. Strain Hxd3 is the first anaerobe shown to grow definitely on saturated hydrocarbons.
Similar content being viewed by others
Abbreviations
- CO dehydrogenase:
-
carbon monoxide dehydrogenase
- DTE:
-
1,4-dithioerythritol
- Tris:
-
tris(hydroxymethyl)-aminomethane
References
Alperin MJ, Reeburgh WS (1985) Inhibition experiments on anaerobic methane oxidation. Appl Environ Microbiol 50:940–945
Atlas RM (1981) Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol Rev 45:180–209
Azoulay E, Chouteau J, Davidovics G (1963) Isoelement et characterisation des enzymes responsables de l'oxydation des hydrocarbures. Biochim Biophys Acta 77:554–567
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Belyayev SS, Laurinavichus KS, Obraztsova AYa, Gorlatov SN, Ivanov MV (1982) Microbiological processes in the critical zone of injection wells. Mikrobiologiya (USSR) 51:997–1001
Birch LD, Bachofen R (1988) Microbial production of hydrocarbons. In: Rehm H-J (ed) Biotechnology, vol. 6b. Verlag Chemie, Weinheim, pp 71–99
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72:248–254
Brandis-Heep A, Gebhardt N, Thauer RK, Widdel F, Pfennig N (1983) Anaerobic acetate oxidation to CO2 by Desulfobacter postgatei. 1. Demonstration of all enzymes required for the operation of the citric acid cycle. Arch Microbiol 136:222–229
Britton LN (1984) Microbial degradation of aliphatic hydrocarbons. In: Gibson TD (ed) Microbial degradation of organic compounds. Marcel Dekker, New York Basel, pp 89–129
Bryant MP (1972) Commentary on the Hungate technique for culture of anaerobic bacteria. Am J Clin Nutr 25:1324–1328
Brysch K, Schneider C, Fuchs G, Widdel F (1987) Lithoautotrophic growth of sulfate-reducing bacteria, and description of Desulfobacterium autotrophicum gen. nov., sp. nov. Arch Microbiol 148:264–274
Bühler M, Schindler J (1984) Aliphatic hydrocarbons. In: Kieslich K (ed) Biotechnology, vol 6a. Verlag Chemie, Weinheim, pp 329–385
Cheesbrough TM, Kolattukudy PE (1984) Alkane biosynthesis by decarbonylation of aldehydes catalyzed by a particulate preparation from Pisum sativum. Proc Natl Acad Sci USA 81:6613–6617
Chouteau J, Azoulay E, Senez JC (1962) Anaerobic formation of n-hept-1-ene from n-heptane by resting cells of Pseudomonas aeruginosa. Nature 194:576–578
Cline JD (1969) Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14:454–458
Cord-Ruwisch R, Kleinitz W, Widdel F (1986) Sulfatreduzierende Bakterien in einem Erdölfeld — Arten und Wachstumsbedingungen. Erdöl, Erdgas, Kohle, 102. Jahrg:281–289
Cord-Ruwisch R, Kleinitz W, Widdel F (1987) Sulfate-reducing bacteria and their activities in oil production. J Petrol Technol, January 1987:97–106
Davis JB, Yarbrough HF (1966) Anaerobic oxidation of hydrocarbons by Desulfovibrio desulfuricans. Chem Geol 1:137–144
Dawson RMC, Elliott DC, Elliott WH, Jones KM (1986) Data for biochemical research. Clarendon Press, Oxford, p 130
Devereux R, Delaney M, Widdel F, Stahl DA (1989) Natural relationships among sulfate-reducing eubacteria. J Bacteriol 171:6689–6695
Dolfing J, Zeyer J, Binder-Eicher P, Schwarzenbach RP (1990) Isolation and characterization of a bacterium that mineralizes toluene in the absence of molecular oxygen. Arch Microbiol 154:336–341
Giger W, Schaffner C, Wakeham SG (1980) Aliphatic and olefinic hydrocarbons in recents of Greifensee, Switzerland. Geochim Cosmochim Acta 44:119–129
Grbié-Galié D, Vogel TM (1987) Transformation of toluene and benzene by mixed methanogenic cultures. Appl Environ Microbiol 53:254–260
Iizuka H, Iida M, Fujita S (1969) Formation of n-decene-1 from n-decane by resting cells of Candida rugosa. Z Allg Mikrobiol 9:223–226
Iversen N, Jørgensen BB (1985) Anaerobic methane oxidation rates at the sulfate-methane transition in marine sediments from Kattegat and Skagerrak (Denmark). Limnol Oceanogr 30:944–955
Jobson AM, Cook FD, Westlake DWS (1979) Interaction of aerobic and anaerobic bacteria in petroleum biodegradation. Chem Geol 24:355–365
Loveley DR, Baedecker MJ, Lonergan DJ, Cozzarelli IM, Phillips EJ, Siegel DI (1989) Oxidation of aromatic contaminants coupled to microbial iron reduction. Nature 339:297–300
Möller-Zinkhan D, Börner G, Thauer RK (1989) Function of methanofuran, tetrahydromethanopterin, and coenzyme F420 in Archaeoglobus fulgidus. Arch Microbiol 152:362–368
Novelli GD, ZoBell CE (1944) Assimilation of petroleum hydrocarbons by sulfate-reducing bacteria. J Bacteriol 47:447–448
Orr W (1974) Changes in sulfur content and isotopic ratios of sulfur during petroleum maturation—study of Big Horn basin Paleozoic oils. Am Assoc Petrol Geol Bull 58:2295–2318
Parekh VR, Traxler RW, Sobek JM (1977) n-Alkane oxidation enzymes of a pseudomonad. Appl Environ Microbiol 33:881–884
Pfennig N, Widdel F, Trüper HG (1981) The dissimilatory sulfate-reducing bacteria. In: Starr MP, Stolp H, Trüper HG, Balows A, Schlegel HG (eds) The prokaryotes, vol I. Springer, Berlin Heidelberg New York, pp 926–940
Reeburgh WS, Alperin MJ (1988) Studies on anaerobic methane oxidation. Mitt Geol-Paläont Inst Univ Hamburg 66:367–375
Rosenfeld WD (1947) Anaerobic oxidation of hydrocarbons by sulfate-reducing bacteria. J Bacteriol 54:664–665
Schauder R, Eikmanns B, Thauer RK, Widdel F, Fuchs G (1986) Acetate oxidation to CO2 in anaerobic bacteria via a novel pathway not involving reactions of the citric acid cycle. Arch Microbiol 145:162–172
Schauder R, Widdel F, Fuchs G (1987) Carbon assimilation pathways in sulfate-reducing bacteria. II. Enzymes of a reductive citric acid cycle in the autotrophic Desulfobacter hydrogenophilus. Arch Microbiol 148:218–225
Schink B (1985a) Degradation of unsaturated hydrocarbons by methanogenic enrichment cultures. FEMS Microbiol Ecol 31:69–77
Schink B (1985b) Fermentation of acetylene by an obligate anaerobe, Pelobacter acetylenicus sp. nov. Arch Microbiol 142:295–301
Senez JC, Azoulay E (1961) Déshydrogénation d'hydrocarbures paraffiniques par les suspensions non-proliférantes et les extraits de Pseudomonas aeruginosa. Biochim Biophys Acta 47:307–316
Synowietz C (1983) D'Ans· Lax, Taschenbuch für Chemiker und Physiker, Bd. II. Springer, Berlin Heidelberg New York, pp 997–1038
Swain HM, Somerville HJ (1978) Denitrification during growth of Pseudomonas aeruginosa on octane. J Gen Microbiol 107:103–112
Thauer RK, Möller-Zinkhan D, Spormann A (1989) Biochemistry of acetate catabolism in anaerobic chemotrophic bacteria. Annu Rev Microbiol 43:43–67
Tissot BP, Welte DH (1984) Petroleum formation and occurrence. Springer, Berlin Heidelberg New York
Traxler RW, Bernard JM (1969) The utilization of n-alkanes by P. aeruginosa under conditions of anaerobiosis. I. Preliminary observations. Int Biodeterior Bull 5:21–25
Vogel TM, Grbié-Galié D (1986) Incorporation of oxygen from water into toluene and benzene during anaerobic fermentative transformation. Appl Environ Microbiol 52:200–202
Ward DM, Brock TD (1978) Anaerobic metabolism of hexadecane in sediments. Geomicrobiol J 1:1–9
Weast RC (1989) Handbook of chemistry and physics. CRC Press, Boca Raton, Florida, p D-210
Widdel F (1980) Anaerober Abbau von Fettsäuren und Benzoesäure durch neu isolierte Arten Sulfat-reduzierender Bakterien. Dissertation, Universität Göttingen
Widdel F (1988) Microbiology and ecology of sulfate-and sulfurreducing bacteria. In: Zehnder AJB (ed) Biology of anaerobic microorganisms. Wiley & Sons, New York, pp 469–585
Widdel F, Pfennig N (1984) Dissimilatory sulfate-or sulfur-reducing bacteria. In: Krieg NR, Holt JG (eds) Bergey's manual of systematic bacteriology, vol I. Williams & Wilkins, Baltimore London, pp 663–679
Woese CR (1987) Bacterial evolution. Microbiol Rev 51:221–271
Zehnder AJB, Brock TD (1979) Methane formation and methane oxidation by methanogenic bacteria. J Bacteriol 137:420–432
Zehnder AJB, Brock TD (1980) Anaerobic methane oxidation: occurrence and ecology. Appl Environ Microbiol 39:194–204
Author information
Authors and Affiliations
Additional information
Dedicated to Dr. Ralph S. Wolfe on occasion of his 70th birthday
Rights and permissions
About this article
Cite this article
Aeckersberg, F., Bak, F. & Widdel, F. Anaerobic oxidation of saturated hydrocarbons to CO2 by a new type of sulfate-reducing bacterium. Arch. Microbiol. 156, 5–14 (1991). https://doi.org/10.1007/BF00418180
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00418180