Abstract
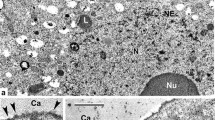
Exocytosis occurring during deposition of secondary wall material was studied by freeze-fracturing ultrarapidly frozen non-plasmolyzed and plasmolyzed tobacco pollen tubes. The secondary wall of tobacco pollen tubes shows a random orientation of microfibrils. This was observed directly on fractures through the tube wall and indirectly as imprints of microfibrils on fracture faces of the plasma membrane of non-plasmolyzed tubes. About half of the plasmatic fracture faces from non-plasmolyzed and plasmolyzed pollen tubes carried hexagonal arrays of intramembraneous particles in between randomly distributed particles. Deposition of secondary wall material was often accompanied by an undulated plasma membrane and the presence of membrane-bound vesicles in invaginations of the plasma membrane, between the plasma membrane and secondary wall and-especially in plasmolyzed tubes-within the secondary wall of tube flanks and wall cap. The findings are discussed in connection with published schemes of membrane behaviour during exocytosis.
Similar content being viewed by others
Abbreviations
- EF:
-
extraplasmatic fracture face
- IMP(s):
-
intramembraneous particle(s)
- PF:
-
plasmatic fracture face
References
Barnabas, A.D., Butler, V., Steinke, T.D. (1982) Zostera capensis Setchell: III. Some aspects of wall ingrowth development in leaf blade epidermal cells. Protoplasma 110, 87–94
Branton, D., Bullivant, S., Gilula, N.B., Karnovsky, M.J., Moor, H., Mühlethaler, K., Northcote, D.H., Packer, L., Satir, B., Satir, P., Speth, V., Staehelin, L.A., Steere R.L., Weinstein R.S. (1975) Freeze-etching nomenclature. Science 190, 54–56
Branton, D., Moor, H. (1964) Fine structure in freeze-etched Allium cepa L. root tips. J. Ultrastruct. Res. 11, 401–411
Brown, R.M., Jr. (1975) The Golgi apparatus and endomembrane system: its role in the biosynthesis, transport, and secretion of cell wall constituents in Pleurochrysis. Port. Acta Biol. 14, 369–384
Burgess, J. (1983) Wall regeneration around isolated protoplasts. Int. Rev. Cytol. Suppl. 16, 55–77
Chandler, D.E., Heuser, J.E. (1979) Membrane fusion during secretion. Corticle granule exocytosis in sea urchin eggs as studied by quick-freeze and freeze-fracture. J. Cell Biol. 83, 91–108
Chandler, D.E., Heuser J.E. (1980) Arrest of membrane fusion events in most cells by quick-freezing. J. Cell Biol. 86, 666–674
Cresti, M., Pacini, E., Ciampolini, F., Sarfatti, G. (1977) Germination and early tube development in vitro of Lycopersicum peruvianum pollen. Planta 136, 239–247
Cresti, M., van Went, J.L. (1976) Callose deposition and plug formation in Petunia pollen tubes in situ. Planta 133, 35–40
Emons, A.M.C. (1985) Plasma membrane rosettes in root hairs of Equisetum hyemale Planta 163, 350–359
Esau, K., Cheadle, V.I., Gill, R.H. (1966) Cytology of differentiating tracheary elements. II. Structures associated with cell surfaces. Am. J. Bot. 53, 765–771
Fowke, L.C., Pickett-Heaps, J.D. (1972) A cytochemical and autoradiographic investigation of cell wall deposition in fiber cells of Marchantia berteroand. Protoplasma 74, 19–32
Frey-Wyssling, A. (1962) Interpretation of the ultratexture in growing plant cell walls. In: Symp. Int. Soc. for Cell Biology, vol. 1: The interpretation of ultrastructure, pp. 307–323, Harris, R.J.C., ed. Academic Press, New York London
Frei, E., Preston, R.D. (1961). Cell wall organization and wall growth in the filamentous green algae Cladophora and Chaetomorpha. I. The basic structure and its formation. Proc. R. Soc. London B, 154, 70–94
Gordon-Kamm, W.J., Steponkus, P.L. (1984) The influence of cold acclimation on the behavior of the plasma membrane following osmotic contraction of isolated protoplasts. Protoplasma 123, 161–173
Gunning, B.E.S., Steer, M.W. (1975) Ultrastructure and the biology of plant cells. Arnold, London
Herrero, M., Dickinson, H.G. (1981) Pollen tube development in Petunia hybrida following compatible and incompatible intraspecific matings. J. Cell Sci. 47, 365–383
Heslop-Harrison, J. (1979) Aspects of the structure, cytochemistry and germination of the pollen of rye (Secale cereale). Ann. Bot. 44, Suppl. 1, 1–47
Iwanami, Y. (1959) Physiological studies of pollen. J. Yokohama Municipal Univ. 116, 1–137
Jensen, W.A., Fisher, D.B. (1970) Cotton embryogenesis: the pollen tube in the stigma and style. Protoplasma 69, 215–235
Kachadorian, W.A., Wade, J.B., Discala, V.A. (1975) Vasopressin: Induced structural change in toad bladder luminal membrane. Science 190, 67–69
Kristen, U., Lockhausen, J. (1983) Estimation of Golgi membrane flow rates in ovary glands of Aptenia cordifolia using cytochalasin B Eur. J. Cell Biol. 29, 262–250
Kroh, M., Knuiman, B. (1982) Ultrastructure of cell wall and plugs of tobacco pollen tubes after chemical extraction of polysaccharides. Planta 154, 241–250
Kroh, M., Knuiman, B., Kudlicka, K. (1983) Effect of reduced turgor pressure on tobacco pollen tubes. In: Fertilization and embryogenesis in ovulated plants, pp. 121–124, Erdelska, O., ed. VEDA, Bratislava
Kudlicka, K., Kroh M., Sassen, M.M.A. (1981) Cell wall regeneration in plasmolyzed pollen tubes of lily and tobacco. Acta Bot. Neerl. 30, 316–317
Larson, D.A. (1965) Fine-structural changes in the cytoplasm of germinating pollen. Am. J. Bot. 52, 139–154
Leonard, R.T., Hodges, T.K. (1980) The plasma membrane: In: The biochemistry of plants, vol. 1: The plant cell, pp. 163–182, Tolbert, N.E., ed. Academic Press, New York London
Mollenhauer, H.H., Morré, D.J. (1966) Golgi apparatus and plant secretion. Annu. Rev. Plant Physiol. 17, 27–46
Moor, H., Mühlethaler, K. (1963) Fine structure in frozen yeast cells. J. Cell Biol. 17, 609–628
Morré, D.J., Mollenhauer H.H. (1983) Dictyosome polarity and membrane differentiation in outer cap cells of the maize root tip. Eur. J. Cell Biol. 29, 126–132
Mueller, S.C. (1982) Cellulose-microfibrils assembly and orientation in higher plant cells with particular reference to seedlings of Zea mays. In: Cellulose and other natural polymer systems, pp. 87–103, Brown R.M., Jr., ed. Plenum Press, New York London
Müller-Stoll, W.R., Lerch, G. (1957) Über Nachweis, Entstehung und Eigenschaften der Kallosebildungen in Pollenschläuchen. Flora 144, 297–334
Northcote, D.H. (1969) Fine structure of cytoplasm, in relation to synthesis and secretion in plant cells. Proc. R. Soc. London B 173, 21–30
Northcote, D.H. (1972) Chemistry of the plant cell wall. Annu. Rev. Plant Physiol. 23, 113–132
Northcote, D.H., Lewis, D.R. (1968) Freeze-etched surfaces of membranes and organelles in the cell of pea root tips. J. Cell Sci. 3, 199–206
Orci, L., Perrelet A., Friends, D.S. (1977) Freeze-fracture of membrane fusions during exocytosis in pancreatic B-cells. J. Cell Biol. 75, 23–30
Palade, G.E. (1975) Intracellular aspects of the process of protein synthesis. Science 189, 347–358
Pickett-Heaps, J.D. (1966) Incorporation of radiactivity into wheat xylem walls. Planta 71, 15–19
Picton, J.M., Steer, M.W. (1983) The effect of cycloheximide on dictyosome activity in Tradescantia pollen tubes determined using cytochalasin D. Eur. J. Cell Biol. 29, 133–138
Plattner, H. (1981) Membrane behavior during exocytosis. Cell Biol. Int. Rep. 5, 435–459
Plattner, H., Bachmann, L. (1982) Cryofixation: a tool in biological ultrastructural research. Int. Rev. Cytol. 79, 237–304
Prat, R., Roland, J.-C. (1971) Etude ultrastructurale des premiers stades de néoformation d'une enveloppe par les protoplastes végétaux separés mécaniquement de leur paroi. C.R. Acad. Sci. 273, 165–168
Preston R.D. (1974) The physical biology of plant cell wall. Chapman & Hall, London
Reiss, H.-D., Herth W. (1979) Calcium ionophore A 23187 affects localized wall secretion in the tip region of pollen tubes of Lilium longiflorum. Planta 145, 225–232
Reiss, H.-D., Herth, W. (1980) Broad-range effects of ionophore X-537A on pollen tubes of Lilium longiflorum. Planta 147, 295–301
Reiss, H.D., Herth, W., Schnepf, E. (1985) Plasma-membrane “rosettes” are present in the lily pollen tube. Naturwissen-schaften 72, 276
Robards, A. W. (1969) Particles associated with developing plant cell walls. Planta 88, 376–379
Robenek, H., Peveling, E. (1977) Ultrastructure of the cell wall regeneration of isolated protoplasts of Skimmia japonica Thunb. Planta 136, 135–145
Robinson, D.G., Cummins, W.R. (1976) Golgi apparatus secretion in plasmolyzed Pisum sativum. Protoplasma 90, 369–379
Robinson, D.G., Kristen, U. (1982) Membrane flow via the Golgi apparatus of higher plant cells. Int. Rev. Cytol. 77, 89–127
Roelofsen, P.A. (1959) The plant cell wall. Gebrüder Bornträger, Berlin
Roland, J.-C. (1973) The relationship between the plasmalemma and the cell wall. Int. Rev. Cytol. 36, 45–92
Roland, J.-C. (1982) Implication de l'appareil vacuolaire dans les phénomène d'extension cellulaire et de morphogenèse pariétale. Bull. Sco. Bot. Fr. 129, 55–61
Rosen, W.G., Gawlik, S.R., Dashek, W.V., Siegesmund, K.A. (1964) Fine structure and cytochemistry of Lilium pollen tubes. Am. J. Bot. 51, 61–71
Ryser, U. (1979) Cotton fibre differentiation: occurrence and distribution of coated and smooth vesicles during primary and secondary wall formation. Protoplasma 98, 223–239
Sassen, M.M.A. (1964) Fine structure of Petunia pollen grain and pollen tube. Acta Bot. Neerl. 13, 175–181
Sassen, M.M.A., Pluymaekers, H.J., Meekes, H.Th.H.M., Jong-Emons, A.M.C. (1981) Cell wall texture in root hairs. In: Cell Walls '81 (Proc. 2nd Cell Wall Meet.), pp. 189–197, Robinson, D.G., Quader, H., eds. Wissenschaftliche Verlagsgesellschaft, Stuttgart
Schmidt, W., Patzak, A., Lingg, G., Winkler H., Plattner, H. (1983) Membrane events in adrenal chromaffin cells during eoxcytosis: a freeze-etching analysis after rapid cryofixation. Eur. J. Cell Biol. 32, 31–37
Schnabl, H., Vienken, J., Zimmermann, U. (1980) Regular arrays of intramembraneous particles in the plasmalemma of guard cell and mesophyll cell protoplasts of Vicia faba. Planta 148, 231–237
Schnepf, E. (1969) Sekretion und Exkretion bei Pflanzen. Protoplasmatologia 8, 1–181
Schnepf, E. (1974) Microtubules and cell wall formation. Portug. Acta Biol. 14, 451–462
Schnepf, E., Busch, J. (1976) Morphology and kinetics of slime secretion in glands of Mimulus tilingii. Z. Pflanzenphysiol. 79, 61–71
Sievers, A., Schnepf, E. (1981) Morphogenesis and polarity of tubular cells with tip growth. In: Cell biology monographs, vol. 8: Cytomorphogenesis in plants, pp. 265–299, Kiermayer, O. ed. Springer, Wien New York
Schröter, K., Sievers, A. (1971) Wirkung der Turgorreduktion auf den Golgi-Apparat und die Bildung der Zellwand bei Wurzelhaaren. Protoplasma 72, 203–211
Volkmann, D. (1982) Structural differentiation of membranes involved in the secretion of polysaccharide slime by root cap cells of cress (Lepidium sativum L.) 151, 180–188
Volkmann D. (1984) The plasma membrane of growing root hairs is composed of zones of local differentiation. Planta 162, 392–403
Westafer, J.M., Brown, R.M.Jr. (1976) Electron microscopy of the cotton fibre: new observations on cell wall formation. Cytobios 15, 111–138
Whaley, W.G., Dauwalder, M. (1979) The Golgi apparatus, the plasma membrane, and functional integration. Int. Rev. Cytol. 58, 199–245
Wilkinson, M.J., Northcote, D.H. (1980) Plasma membrane ultrastructure during plant protoplast plasmolysis, isolation and wall regeneration: a freeze-fracture study. J. Cell Sci. 42, 401–415
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kroh, M., Knuiman, B. Exocytosis in non-plasmolyzed and plasmolyzed tobacco pollen tubes. Planta 166, 287–299 (1985). https://doi.org/10.1007/BF00401164
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00401164