Abstract
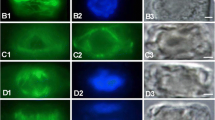
In a study of pollen development in Gasteria verrucosa, the changes in the spatial organization of microtubules were related to the processes of cell division, nuclear movement and cytomorphogenesis. Sections of polyethylene-glycol-embedded anthers of G. verrucosa were processed immunocytochemically to record the structure and succession of fluorescently labeled microtubular configurations. Using microspectrophotometric measurements the relative quantity of tubulin in microtubules per unit of cytoplasm was determined. Cell dimensions and nuclear positions were measured to relate changes in cell shape and nuclear movements to microtubular configurations. Microtubules were detected in the different cells during microsporogenesis and microgametogenesis. In microspore mother cells which are approximately isodiametric at interphase, microtubules were predominantly arranged in a criss-cross pattern. The microtubules probably function as a flexible cytoskeleton which sustains the integrity of the cytoplasm. Bundles of microtubules were observed in the microspores, in the generative cells and during nuclear division, where they functioned in establishing and maintaining cell and spindle shapes. Microtubules radiating from nuclear membranes appeared to fix the nucleus in position. In prophase of meiosis and after microspore mitosis, periods a high fluorescence intensity were distinguished indicating a variation in the quantity of microtubules.
Similar content being viewed by others
Abbreviations
- MT:
-
microtubule
References
Bassel, A.R. Kuehnert, C.C., Miller, J.H. (1981) Nuclear migration and asymmetric cell division in Onoclea sensibilis spores: an ultrastructural and cytochemical study. Am. J. Bot. 68, 350–360
Brown, R.C., Lemmon, B.E. (1982) Ultrastructure of meiosis in the moss Rhynchostegium serrulatum. I. Prophasic microtubules and spindle dynamics. Protoplasma 110, 23–33
Burgess, J. (1970) Microtubules and cell division in the microspore of Dactylorchis fuschii. Protoplasma 69, 253–264
Ciampolini, F., Cresti, M., De Dominicis, V., Garavito, R.M., Sarfatti, G. (1980) Intranuclear cristalloids in leaves and styles of Linaria vulgaris Mill. J. Ultrastruct. Res. 71, 14–21
Cresti, M., Ciampolini, F., Kapil, R.N. (1984) Generative, cells of some angiosperms with particular emphasis on their microtubules. J. Submicrosc. Cytol. 16, 317–326
Dickinson, H.G., Sheldon, J.M. (1984) A radial system of microtubules extending between the nuclear envelope and the plasma membrane during early male haplophase in flowering plants. Planta 161, 86–90
Gerassimova-Navashina, H. (1957) On some cytological principles underlying double fertilization. Phytomorphology 57, 153–167
Johnson, G.D., C. Nogueira Araujo, G.M. de (1981) A simple method of reducing the fading of immunofluorescence during microscopy. J. Immunol. Methods, 43, 349–350
Keijzer, C.J. (1983) Hydration changes during anther development. In: Pollen: Biology and implications for plant breeding, pp. 197–201, Mulcahy, D.L., Ottaviano, E., eds., Elsevier Biomedical, New York Amsterdam Oxford
Keijzer, C.J. (1985a) Anther development of Gasteria. In: Sexual reproduction in seed plants, ferns and mosses, p. 20, Willemse, M.T.M., Van Went, J.L., eds. Pudoc, Wageningen
Keijzer, C.J. (1985b) The functions, of the endothecium. Acta Bot. Neerl. 34, in press.
Kreis, T.E., Birchmeier, W. (1982) Microinjection of fluorescently labeled proteins into living cells with emphasis on cytoskeletal proteins. Int. Rev. Cytol. 75, 209–227
Ledbetter, M.C., Porter, K.R. (1963) A “microtubule” in plant fine structure J. Cell. Biol. 19, 239–250
Lloyd, C.W. ed. (1982) The cytoskeleton in plant growth and development. Academic Press, London
Lloyd, C.W., Slabas, A.R., Powell, A.J., MacDonald, G., Badley, R.A. (1979) Cytoplasmic microtubules of higher plant cells visualized with anti-tubulin antibodies. Nature 279, 239–241
McIntosh, J.R. (1984) Mechanisms of mitosis. Trend Biochem. Sci. 9, 195–198
Osborn, M., Weber, K. (1982) Immunofluorescence and immunocytochemical procedures with affinity purified antibodies: Tubulin-containing structures. In: Methods in cell biology, vol. 2: The cytoskeleton, part A, pp. 97–132, Wilson, L., ed. Academic Press, London
Owens, S.J., Westmuckett, A.D. (1983) The structure and development of the generative cell wall in Gibasis karwinskyana, G. venustula and Tradescantia blossfeldiana (Commelinaceae). In: Pollen: Biology and implications for plant breeding, pp. 149–157, Mulcahy, D.L., Ottaviano, E., eds. Elsevier Biomedical, New York Amsterdam Oxford
Sanger, J.M., Jackson, W.T. (1971) Fine structure study of pollen development in Haemanthus katherinae Baker. II. Microtubules and elongation of the generative cells. J. Cell Sci. 8, 303–315
Shelanski, M.L., Gaskin, F. Cantor, C.R. (1973) Microtubule assembly in the absence of added nucleotides. Proc. Natl. Acad. Sci. 70, 765–768
Sheldon, J.M., Dickinson, H.G. (1983) Determination of patterning in the pollen wall of Lilium henryi. J. Cell Sci. 63, 191–208
Spurr, A.R. (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43
Van Lammeren, A.A.M. (1982) Detection of cytoplasmic and spindle microtubules in plant cells by indirect immunofluorescence. In: Proc. 23rd Dutch Fed. Meeting, Amsterdam, p. 256, Dutch Foundation Fed. Med. Sci. Soc.
Van Lammeren, A.A.M. (1985) Microtubular configurations during endosperm and pollen development in Zea and Gasteria as visualized by immunofluorescence. In: Sexual reproduction in seed plants, ferns and mosses, pp. 59–63, Willemse, M.T.M. Van Went, J.L., eds. Pudoc, Wageningen
Willemse, M.T.M. (1971) Morphological and quantitative changes in the population of cell organelles during microsporogenesis of the Pinus sylvestris L III. Morphological changes during the tetrad stage and in the young microspore. A quantitative approach to the changes in the population of cell organelles. Acta Bot. Neerl. 20, 498–523
Willemse, M.T.M. (1972) Morphological and quantitative changes in the population of cell organelles during microsporogenesis of Gasteria verrucosa. Acta Bot. Neerl. 21, 17–31
Willemse, M.T.M. (1981) Polarity during megasporogenesis and megagametogenesis. Phytomorphology 31, 124–134
Willemse, M.T.M. (1982) Changes in autofluorescence of lignin. In: Cell walls 1981; Proc. 2nd Cell Wall Meeting, Göttingen, April 8th–11th, 1981, pp. 242–250, Robinson, D.G., Quader, H., eds. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Lammeren, A.A.M., Keijzer, C.J., Willemse, M.T.M. et al. Structure and function of the microtubular cytoskeleton during pollen development in Gasteria verrucosa (Mill.) H. Duval. Planta 165, 1–11 (1985). https://doi.org/10.1007/BF00392205
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392205