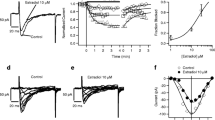
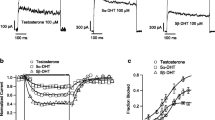
Abstract
Hyperpolarization of patch-perforated GH3 rat anterior pituitary cells in high-K+ Ca2+-free medium reveals an inwardly rectifying K+ current. This current showed potential-dependent activation and inactivation kinetics, complete inactivation during strong hyperpolarization and rectification at depolarized potentials. The current was blocked by millimolar concentrations of external Cs+, Ba2+, Cd2+ and Co2+, but it was almost insensitive to tetraethylammonium, 4-aminopyridine and two dihydropyridines, nisoldipine and nitrendipine. Verapamil and methoxyverapamil produced a strong and reversible inhibition of the current. In the presence of 100 nM thyrotropin-releasing hormone (TRH), the current was reduced. This reduction was increased by holding the cell at more negative potentials and was accompanied by a shift in steady-state voltage dependence of inactivation towards more positive voltages. Furthermore, the current slowly returned to the initial levels upon washout. Treatment of the cell with the protein phosphatase inhibitor okadaic acid increased the magnitude of the inhibition caused by TRH. Moreover, the current did not return towards the control level during a 30-min washout period. It is concluded that protein phosphatases participate in modulation of the GH3 cell inwardly rectifying K+ channels by TRH. Furthermore, these data indicate that either a protein phosphatase or a factor necessary for its activation is lost under whole-cell mode, which could account for the permanent reduction of the current in response to TRH.
Similar content being viewed by others
References
Albert PR, Tashjian AH Jr (1985) Dual actions of phorbol esters on cytosolic free Ca2+ concentrations and reconstitution with ionomycin of acute thyrotropin-releasing hormone responses. J Biol Chem 260:8746–8759
Barros F, Katz GM, Kaczorowski GJ, Vandlen RL, Reuben JP (1985) Calcium currents in GH3 cultured pituitary cells under whole-cell voltage-clamp: inhibition by voltage-dependent potassium currents. Proc Natl Acad Sci USA 82:1108–1112
Barros F, Kaczorowski GJ, Katz GM, Vandlen RL, Reuben JP (1986) Application of whole-cell voltage clamp in the study of neuroendocrine cells. In: Geller HM (ed) Electrophysiological techniques in pharmacology. Modern methods in pharmacology, vol 3. Liss, New York, pp 149–168
Barros, F, Delgado LM, Maciá C, de la Peña P (1991) Effects of hypothalamic peptides on electrical activity and membrane currents of “patch perforated” clamped GH3 anterior pituitary cells. FEBS Lett 279:33–37
Bauer CK, Meyerhof W, Schwarz JR (1990) An inward-rectifying K+ current in clonal rat pituitary cells and its modulation by thyrotropin-releasing hormone. J Physiol (Lond) 429:169–189
Bialojan C, Takai A (1988) Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Biochem J 256:283–290
Bjoro T, Sand O, Ostberg BG, Gordeladze JO, Torjesen P, Gautvik KM, Haug E (1990) The mechanisms by which vasoactive intestinal peptide (VIP) and thyrotropin releasing hormone (TRH) stimulate prolactin release from pituitary cells. Biosci Rep 10:189–199
Cohen P (1989) The structure and regulation of protein phosphatases. Annu Rev Biochem 58:453–508
Cohen P, Holmes FB, Tsukitani Y (1990) Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem. Sci 15:98–102
Drummond AH (1989) The pituitary gland and its constituent cells. In: Michell RH, Drummond AH, Bushfield M, MacPhee CH (eds) Inositol lipids in cell signalling. Academic Press, London, pp 355–375
Drummond AH, Bushfield M, MacPhee CH (1984) Thyrotropin releasing hormone-stimulated [3H]inositol metabolism in GH3 pituitary tumour cells. Studies with lithium. Mol Pharmacol 25:201–208
Dubinsky JM, Oxford GS (1985) Dual modulation of K channels by thyrotropin releasing hormone in clonal pituitary cells. Proc Natl Acad Sci USA 82:4282–4286
Dufy B, MacDermott A, Barker JL (1986) Rundown of GH3 cell K+ conductance response to TRH following patch recording can be obviated with GH3 cell extract. Biochem Biophys Res Commun 137:388–396
Dufy B, Jaken S, Barker JL (1987) Intracellular Ca2+-dependent protein kinase C activation mimics delayed effects of thyrotropin-releasing hormone on clonal pituitary cell excitability. Endocrinology 121:793–802
Gammon CM, Oxford GS, Allen AC, McCarthy KD, Morell P (1989) Diacylglycerol modulates action potential frequency in GH3 pituitary cells: correlative biochemical and electrophysiological studies. Brain Res 479:217–224
Gershengorn MC (1986) Mechanism of thyrotropin releasing hormone stimulation of pituitary hormone secretion. Annu Rev Physiol 48:515–526
Gershengorn MC, Paul ME (1986) Evidence for tight coupling of receptor occupancy by thyrotropin releasing hormone to phospholipase C-mediated phosphoinositide hydrolysis in rat pituitary cells: use of chlordiazepoxide as a competitive antagonist. Endocrinology 119:833–839
Gordeladze JO, Bjoro T, Ostberg BC, Sand O, Torjesen P, Haug E, Gautvik KM (1988) Phorbol esters and thyroliberin have distinct actions regarding stimulation of prolactin secretion and activation of adenylate cyclase in rat pituitary tumour cells (GH4C1 cells). Biochem Pharmacol 37:3133–3138
Gordelazde JO, Bjoro T, Torjesen PA, Ostberg BC, Haug E, Gautvik KM (1989) Protein kinase C stimulates adenylate cyclase activity in prolactin-secreting rat adenoma (GH4C1) pituicytes by inactivating the inhibitory GTP-binding protein Gi Eur J Biochem 183:397–406
Horn R, Marty A (1988) Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol 92:145–159
Korn SJ, Horn R (1989) Influence of sodium-calcium exchange on calcium current rundown and the duration of calcium-dependent chloride currents in pituitary cells, studied with whole cell and perforated patch recording. J Gen Physiol 94:789–912
Kramer RH, Kaczmarek LK, Levitan ES (1991) Neuropeptide inhibition of voltage-gated calcium channels mediated by mobilization of intracellular calcium. Neuron 6:557–563
Lang DG, Ritchie AK (1987) Large and small conductance calcium-activated potassium channels in the GH3 anterior pituitary cell line. Pflügers Arch 410:614–622
Lang DG, Ritchie AK (1990) Tetraethylammonium blockade of apamin-sensitive and insensitive Ca2+-activated K+ channels in a pituitary cell line. J Physiol (Lond) 425:117–132
Lang DG, Ritchie AK (1990) Tetraethylammonium ion sensitivity of a 35-pS Ca2+ activated K+ channel in GH3 cells that is activated by thyrotropin-releasing hormone. Pflügers Arch 416:704–709
Levitan ES, Kramer RH (1990) Neuropeptide modulation of single calcium and potassium channels detected with a new patch clamp configuration. Nature 348:545–547
Martin TFJ, Kowalchyk JA (1984) Evidence for the role of calcium and diacylglycerol as dual second messengers in thyrotropin-releasing hormone action: involvement of Ca2+. Endocrinology 115:1527–1536
Martin TFJ, Hsieh K-P, Porter BW (1990) The sustained second phase of hormone-stimulated diacylglycerol accumulation does not activate protein kinase C in GH3 cells. J Biol Chem 265:7623–7631
Ostberg BC, Sand O, Bjoro T, Haug E (1986) The phorbol ester TPA induces hormone release and electrical activity in clonal rat pituitary cells. Acta Physiol Scand 126:517–524
Oxford GS, Wagoner PK (1989) The inactivating K+ current in GH3 pituitary cells and its modification by chemical reagents. J Physiol (Lond) 410:587–612
Ozawa S, Sand O (1986) Electrophysiology of excitable endocrine cells. Physiol Rev 66:887–952
Schlegel W, Roduit C, Zahnd G (1981) Thyrotropin releasing hormone stimulates metabolism of phosphatidyl inositol in GH3 cells. FEBS Lett 134:47–49
Simasko SM (1991) Reevaluation of the electrophysiological actions of thyrotropin-releasing hormone in a rat pituitary cell line (GH3). Endocrinology 128:2015–2026
White RE, Schonbrunn A, Armstrong DL (1991) Somatostatin stimulates Ca2+-activated K+ channels through protein dephosphorylation. Nature 351:570–573
Winiger BP, Schlegel W (1988) Rapid transient elevations of cytosolic calcium triggered by thyrotropin releasing hormone in individual cells of the pituitary line GH3B6. Biochem J 255:161–167
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barros, F., Delgado, L.M., del Camino, D. et al. Characteristics and modulation by thyrotropin-releasing hormone of an inwardly rectifying K+ current in patch-perforated GH3 anterior pituitary cells. Pflugers Arch. 422, 31–39 (1992). https://doi.org/10.1007/BF00381510
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00381510