Abstract
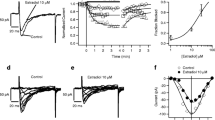
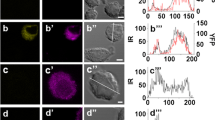
Androgens produce nongenomic effects in several cells by different mechanisms, including ion channel modulation. Adenohypophyseal cells express several K+ channels, including voltage and Ca2+-dependent K+ (BK) channels, which might be the target of androgens to modulate cellular action potentials and hormonal secretion. Androgen effects were studied in GH3 cells (from anterior pituitary rat tumor) by means of the patch-clamp technique. Cells were continuously perfused with saline solution, in the absence or presence of the androgens studied, while applying 40 mV pulses of 400 ms from a holding potential of −60 mV in whole-cell configuration with nystatin-perforated patches. Androgens reversibly blocked noninactivating K+ currents in a concentration-dependent manner without a latency period and with an order of efficacy of: 5β-dihydrotestosterone (DHT)>testosterone>5α-DHT. RT-PCR showed two isoforms of the α-pore forming subunits of BK channels. These channels are responsible for one third of the noninactivating current, according to the blockade of paxilline, a selective BK antagonist. Androgens seem to directly interact with BK channels since they were blocked in excised inside-out patches and independent of the whole-cell configuration and the NO-cGMP-dependent pathway. Testosterone, but not 5α- or 5β-DHT, increased BK currents in HEK-293 cells overexpressing the short isoform, suggesting a cellular selectivity based on the α-subunits. The effect on noninactivating currents may be responsible for the decrease of spontaneous action potential frequency. Long-term cellular incubation with testosterone did not modify noninactivating currents density in GH3 cells. It is remarkable that 5β-DHT, a reductase metabolite with weak androgenic activity, was the most efficient blocker.








Similar content being viewed by others
References
Akaike N, Harata N (1994) Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol 44(5):433–473
Balthazart J, Schumacher M, Malacarne G (1984) Relative potencies of testosterone and 5 alpha-dihydrotestosterone on crowing and cloacal gland growth in the Japanese quail (Coturnix coturnix japonica). J Endocrinol 100(1):19–23
Barros F, Delgado LM, Macia C, de la Pena P (1991) Effects of hypothalamic peptides on electrical activity and membrane currents of 'patch perforated' clamped GH3 anterior pituitary cells. FEBS Lett 279(1):33–37
Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B (2006) BKCa-Cav channel complexes mediate rapid and localized Ca2+−activated K+ signaling. Science 314(5799):615–620
Brunton PJ, Sausbier M, Wietzorrek G, Sausbier U, Knaus HG, Russell JA, Ruth P, Shipston MJ (2007) Hypothalamic-pituitary-adrenal axis hyporesponsiveness to restraint stress in mice deficient for large-conductance calcium- and voltage-activated potassium (BK) channels. Endocrinology 148(11):5496–5506
Carani C, Granata AR, Fustini MF, Marrama P (1996) Prolactin and testosterone: their role in male sexual function. Int J Androl 19(1):48–54
Carter JN, Tyson JE, Tolis G, Van Vliet S, Faiman C, Friesen HG (1978) Prolactin-screening tumors and hypogonadism in 22 men. N Engl J Med 299(16):847–852
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162(1):156–159
Cohen J (1988) Statistical power analysis for the behavioral sciences. Lawrence Erlbaum Associates, Hillsdale
Deenadayalu VP, White RE, Stallone JN, Gao X, Garcia AJ (2001) Testosterone relaxes coronary arteries by opening the large-conductance, calcium-activated potassium channel. Am J Physiol Heart Circ Physiol 281(4):H1720–H1727
Deenadayalu V, Puttabyatappa Y, Liu AT, Stallone JN, White RE (2011) Testosterone-induced relaxation of coronary arteries: activation of BKCa channels via the cGMP-dependent protein kinase. Am J Physiol Heart Circ Physiol 302(1):H115–H123
Denef C (1983) 5 alpha-dihydrotestosterone formation and its functional significance in rat anterior pituitary, subpopulations of gonadotrophs and cell cultures. J Steroid Biochem 19(1A):235–239
Fakler B, Adelman JP (2008) Control of K(Ca) channels by calcium nano/microdomains. Neuron 59(6):873–881
Fernandes VS, Barahona MV, Recio P, Martinez-Saenz A, Ribeiro AS, Contreras C, Martinez AC, Bustamante S, Carballido J, Garcia-Sacristan A, Prieto D, Hernandez M (2012) Mechanisms involved in testosterone-induced relaxation to the pig urinary bladder neck. Steroids 77(5):394–402
Finkelstein JS, O’Dea LS, Whitcomb RW, Crowley WF Jr (1991a) Sex steroid control of gonadotropin secretion in the human male. II. Effects of estradiol administration in normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab 73(3):621–628
Finkelstein JS, Whitcomb RW, O’Dea LS, Longcope C, Schoenfeld DA, Crowley WF Jr (1991b) Sex steroid control of gonadotropin secretion in the human male. I. Effects of testosterone administration in normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab 73(3):609–620
Frye CA, Edinger K, Sumida K (2008) Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology 33(5):1049–1061
Furukawa T, Kurokawa J (2007) Regulation of cardiac ion channels via non-genomic action of sex steroid hormones: implication for the gender difference in cardiac arrhythmias. Pharmacol Ther 115(1):106–115
Gunzel-Apel AR, Seefeldt A, Eschricht FM, Urhausen C, Kramer S, Mischke R, Hoppen HO, Beyerbach M, Koivisto M, Dieleman SJ (2009) Effects of gonadectomy on prolactin and LH secretion and the pituitary-thyroid axis in male dogs. Theriogenology 71(5):746–753
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391(2):85–100
Horn R, Marty A (1988) Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol 92(2):145–159
Hu XQ, Zhang L (2012) Function and regulation of large conductance Ca(2+)-activated K+ channel in vascular smooth muscle cells. Drug Discov Today 17(17–18):974–987
Jamali K, Naylor BR, Kelly MJ, Ronnekleiv OK (2003) Effect of 17beta-estradiol on mRNA expression of large- conductance, voltage-dependent, and calcium-activated potassium channel alpha and beta subunits in guinea pig. Endocrine 20(3):227–237
Kelly DM, Jones TH (2013) Testosterone: a vascular hormone in health and disease. J Endocrinol 217(3):R47–R71
Korovkina VP, Brainard AM, Ismail P, Schmidt TJ, England SK (2004) Estradiol binding to maxi-K channels induces their down-regulation via proteasomal degradation. J Biol Chem 279(2):1217–1223
Lang DG, Ritchie AK (1987) Large and small conductance calcium-activated potassium channels in the GH3 anterior pituitary cell line. Pflugers Arch 410(6):614–622
Lephart ED (1993) Pituitary and brain 5alpha-reductase messenger RNA levels in control, castrated, and dihydrotestosterone-treated rats. Mol Cell Neurosci 4(6):526–531
Lin MT, Hessinger DA, Pearce WJ, Longo LD (2006) Modulation of BK channel calcium affinity by differential phosphorylation in developing ovine basilar artery myocytes. Am J Physiol Heart Circ Physiol 291(2):H732–H740
Martin LJ, Siliart B, Dumon HJ, Nguyen P (2006) Spontaneous hormonal variations in male cats following gonadectomy. J Feline Med Surg 8(5):309–314
Michels G, Hoppe UC (2008) Rapid actions of androgens. Front Neuroendocrinol 29(2):182–198
Ohno A, Ohya S, Yamamura H, Imaizumi Y (2009) Gender difference in BK channel expression in amygdala complex of rat brain. Biochem Biophys Res Commun 378(4):867–871
Ouadid-Ahidouch H, Roudbaraki M, Ahidouch A, Delcourt P, Prevarskaya N (2004) Cell-cycle-dependent expression of the large Ca2+−activated K+ channels in breast cancer cells. Biochem Biophys Res Commun 316(1):244–251
Perusquia M, Stallone JN (2010) Do androgens play a beneficial role in the regulation of vascular tone? Nongenomic vascular effects of testosterone metabolites. Am J Physiol Heart Circ Physiol 298(5):H1301–H1307
Pitteloud N, Quinton R, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W (2007) Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 117(2):457–463
Rochira V, Zirilli L, Genazzani AD, Balestrieri A, Aranda C, Fabre B, Antunez P, Diazzi C, Carani C, Maffei L (2006) Hypothalamic-pituitary-gonadal axis in two men with aromatase deficiency: evidence that circulating estrogens are required at the hypothalamic level for the integrity of gonadotropin negative feedback. Eur J Endocrinol 155(4):513–522
Roselli CE, Abdelgadir SE, Resko JA (1997) Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull 44(4):351–357
Sanchez M, McManus OB (1996) Paxilline inhibition of the alpha-subunit of the high-conductance calcium-activated potassium channel. Neuropharmacology 35(7):963–968
Seminara SB (2006) Mechanisms of disease: the first kiss-a crucial role for kisspeptin-1 and its receptor, G-protein-coupled receptor 54, in puberty and reproduction. Nat Clin Pract Endocrinol Metab 2(6):328–334
Stojilkovic SS, Tabak J, Bertram R (2010) Ion channels and signaling in the pituitary gland. Endocr Rev 31(6):845–915
Takimoto K, Fomina AF, Gealy R, Trimmer JS, Levitan ES (1993) Dexamethasone rapidly induces Kv1.5 K+ channel gene transcription and expression in clonal pituitary cells. Neuron 11(2):359–369
Tang F (1991) Endocrine control of hypothalamic and pituitary met-enkephalin and beta-endorphin contents. Neuroendocrinology 53(Suppl 1):68–76
Tobin VA, Canny BJ (1998) The regulation of gonadotropin-releasing hormone-induced calcium signals in male rat gonadotrophs by testosterone is mediated by dihydrotestosterone. Endocrinology 139(3):1038–1045
Torres YP, Morera FJ, Carvacho I, Latorre R (2007) A marriage of convenience: beta-subunits and voltage-dependent K+ channels. J Biol Chem 282(34):24485–24489
Turner RW, Anderson D, Zamponi GW (2011) Signaling complexes of voltage-gated calcium channels. Channels (Austin) 5(5):440–448
Viau V, Meaney MJ (2004) Testosterone-dependent variations in plasma and intrapituitary corticosteroid binding globulin and stress hypothalamic-pituitary-adrenal activity in the male rat. J Endocrinol 181(2):223–231
Wilson EM, French FS (1976) Binding properties of androgen receptors. Evidence for identical receptors in rat testis, epididymis, and prostate. J Biol Chem 251(18):5620–5629
Acknowledgments
The work was supported by grants from the University of Oviedo (SV-UNOV-09-MA and SV-UNOV-10-MA2), Spain, and Merck RL, Rahway, NJ, USA. Usama Bilal was recipient of a Collaborative Student Scholarships in University Departments for the academic year 2010–2011, from the Ministerio de Educación, Spain.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lorena Suárez and Usama Bilal contributed equally to this work.
Rights and permissions
About this article
Cite this article
Suárez, L., Bilal, U., Bordallo, J. et al. Androgens block outward potassium currents and decrease spontaneous action potentials in GH3 cells. Naunyn-Schmiedeberg's Arch Pharmacol 388, 67–78 (2015). https://doi.org/10.1007/s00210-014-1057-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-014-1057-2