Abstract
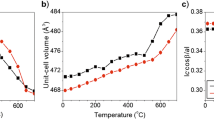
Activity-composition data have been used to obtain excess entropies and enthalpies of mixing for almandine-grossular and Mg-rich pyrope-grossular solid solutions. Excess free energies of Fe-rich almandine-grossular garnets are negative and imply the formation of a stable garnet compound with a different structure from the end members in this series.
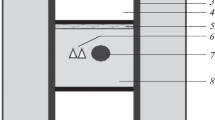
X-ray investigations of natural and synthetic calcium-poor aluminosilicate garnets indicate a lower space group symmetry than Ia3d, that of the end members. The most probable space group is I213 in which there are two crystallographically distinct 8-coordinate sites present in equal numbers in the garnet structure.
Excess entropies of mixing for almandine-grossular garnets are asymmetric and can be explained by Fe-Ca ordering in calcium-poor garnets together with Fe positional disorder on sub-sites within the 8-coordinate polyhedra. In the middle of the series and towards the Ca-rich end, a high degree of sub-site disorder with little or no Fe-Ca ordering may be responsible for the high positive entropies. Excess entropies of calcium-poor pyrope-grossular garnets show a similar trend, but are slightly more positive. Excess enthalpies of mixing for Mg-rich pyropegrossular garnets are in good agreement with the high-temperature calorimetric measurements of other workers.
Similar content being viewed by others
References
Cressey G (1978) Exsolution in almandine — pyrope — grossular garnet. Nature 271:533–534
Cressey G, Schmid R, Wood BJ (1978) Thermodynamic properties of almandine — grossular garnet solid solutions. Contrib Mineral Petrol 67:397–404
Cressey G (1979) Mixing properties of aluminosilicate garnets. Ph D Thesis, Manchester Univ
Cressey G. Structural changes in almandine-rich garnets caused by calcium substitution (in preparation)
Darken LS, Gurry RW (1953) Physical chemistry of metals. New York, McGraw-Hill
Dempsey MJ (1980) Evidence for structural changes in garnet caused by calcium substitution. Contrib Mineral Petrol 71:281–282
Emiliani F, Venturelli G (1972) Sharp compositional zoning in an almandine garnet. Can Mineral 11:464–472
Emiliani F, Zeda O (1974) Sharp compositional variations in garnets from pegmatites in Val Martello (Alto Adige — Italy). Ateneo Parmense Acta Nat 10:299–314
Gibbs GV, Smith JV (1965) Refinement of the crystal structure of synthetic pyrope. Am Mineral 50:2023–2039
Hensen BJ, Schmid R, Wood BJ (1975) Activity-composition relationships for pyrope — grossular garnet. Contrib Mineral Petrol 51:161–166
Iiyama JT, Volfinger M (1976) A model for trace-element distribution in silicate structures. Mineral Mag 40:555–564
Meagher EF (1975) The crystal structures of pyrope and grossularite at elevated temperatures. Am Mineral 60:218–228
Megaw HD (1968) A simple theory of the off-centre displacement of cations in octahedral environments. Acta Crystallogr B24:149–153
Newton RC, Charlu TV, Kleppa OJ (1977) Thermochemistry of high pressure garnets and clinopyroxenes in the system CaO-MgO-Al2O3-SiO2. Geochim Cosmochim Acta 41:369–377
Newton RC, Wood BJ (1980) Volume behaviour of silicate solid solutions. Am Mineral 65:733–745
Novak GA, Gibbs G (1971) The crystal chemistry of the silicate garnets. Am Mineral 56:791–825
Schmid R, Cressey G, Wood BJ (1978) Experimental determination of univariant equilibria using divariant solid solution assemblages. Am Mineral 63:511–515
Smith D, Zientek M (1979) Mineral chemistry and zoning in eclogite inclusions from Colorado Plateau diatremes. Contrib Mineral Petrol 69:119–131
Zemann A, Zemann J (1961) Verfeinerung der Kristallstruktur von synthetischem Pyrop, Mg3Al2(SiO4)3. Acta Crystallogr 14:835–837
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cressey, G. Entropies and enthalpies of aluminosilicate garnets. Contr. Mineral. and Petrol. 76, 413–419 (1981). https://doi.org/10.1007/BF00371483
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00371483