Abstract
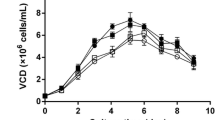
Methylglyoxal is a ketoaldehyde that reacts readily under physiological conditions with biologically relevant ligands, such as amine and sulfhydryl groups. It is produced in mammalian cells primarily as a by-product of glycolysis. The level of glucose, L-glutamine and fetal bovine serum in culture media was found to significantly affect levels of intracellular methylglyoxal in Chinese hamster ovary cells. Medium with 25 mM glucose and 5 mM L-glutamine caused an increase in free methylglyoxal levels of 90 to 100% relative to medium containing 5 mM glucose and 2 mM L-glutamine. Both of these media compositions are representative of those found in commercially available media. Pseudomonas putida glyoxalase I was expressed in Chinese hamster ovary cells to enhance methylglyoxal detoxification. The Chinese hamster ovary cell clones showed an 80 to 90% decrease in free methylglyoxal levels. The colony-forming ability of these cells was compared to wild-type Chinese hamster ovary cells under conditions found to cause elevated methylglyoxal levels. The wild-type cells showed a 10% decrease in colony-forming ability relative to the clones. This decrease was found to be statistically significant (P>0.99) by analysis of variance. The variation in colony-forming ability amongst the clones was statistically insignificant. More importantly, the clones shoed increased colony-forming ability relative to the wild-type cells under conditions of higher methylglyoxal production with fair to good statistical significance (P>0.75 to P>0.95). This result is the first quantifiable evidence that endogenously produced methylglyoxal can negatively affect cell function under conditions found in animal cell culture.
Similar content being viewed by others
Abbreviations
- ANOVA:
-
analysis of variance
- CHO:
-
Chinese hamster ovary cells
- CFA:
-
colony-forming ability
- dhfr :
-
gene for dihydrofolate reductase
- DHAP:
-
dihydroxyacetone phosphate
- FBS:
-
fetal bovine serum
- G-3-P:
-
glyceraldehyde-3-phosphate
- GloI:
-
glyoxalase I
- GloII:
-
glyoxalase II
- GSH:
-
reduced glutathione
- HPLC:
-
high-performance liquid chromatography
- IMDM:
-
Iscove's modified Dulbecco's medium
- MTX:
-
methotrexate
- 2-MQ:
-
2-methylquinoxaline
- 5-MQ:
-
5-methylquinoxaline
- MEM:
-
minimal essential medium
- Pi :
-
inorganic phosphate
- PCA:
-
perchloric acid
- o-PD:
-
o-phenylenediamine
References
Argiles JM (1986) Has acetone a role in the conversion of fat to carbohydrate in mammals? TIBS 11, 61–63.
Atkins TW and Thornalley PJ (1982) Erythrocyte glyoxalase activity in genetically obese (oblob) and streptozotocin diabetic mice. Diabetes Res 11, 125–129.
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K, Albright LM, Coen DM and Varki A (1994) Current Protocols in Molecular Biology, Greene Publishing Associates, Inc., New York.
Box EPG, Hunter WG and Hunter JS (1978) Statistics for experimenters, John Wiley & Sons, Inc. New York.
Chang Y-HD, Grodzinsky AJ and Wang DJC (1995) In-situ removal of ammonium and lactate through electrical means for hybridoma cultures. Biotech Bioeng 47, 308–318.
Chaplen FWR, Fahl WE and Cameron DC (1996a) Method for determination of free extracellular and intracellular methylglyoxal in animal cells grown in culture. Anal Biochem 238, 171–178.
Chaplen FWR, Fahl WE and Cameron DC (1996b) Detection of methylglyoxal as a degradation product of DNA and nucleic acids components treated with strong acid. Anal Biochem 236, 262–269.
Chaplen FWR (1996) Analysis of Methylglyoxal Metabolism in Mammalian Cell Culture, PhD Thesis, University of Wisconsin-Madison, Madison, Wisconsin.
Chaudhary AK, Nokubo M, Reddy GR, Yeola SN, Morrow JD, Blair IA and Marnett LJ (1994) Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science 265, 1580–1582.
Griffiths JB and Racher AJ (1994) Cultural and physiological factors affecting expression of recombinant proteins. Cytotechnology 15, 3–9.
Hu W-S and Himes VB (1989) Stoichiometric considerations of mammalian cell metabolism in bioreactors. In: Bioproducts and Bioprocesses, Feichter A, Okada H, and Tanner RD (eds), Springer-Verlag, Berlin, pp. 33–45.
Jocelyn PC (1973) Biochemistry of the SH group, Academic Press, New York, 1972. Krymkiewwicz, N. Reaction of methylglyoxal with nucleic acids. FEBS Lett 29, 51–54.
Lo TWC, Westwood ME, McLellan AC, Selwood T and Thornalley PJ (1994) Binding and modification of proteins by methylglyoxal under physiological conditions. J Biol Chem 269, 32299–32305.
McLellan AC, Thornalley PJ, Benn J and Sonksen PH (1994) The glyoxalase system in clinical diabetes mellitus and correlation with clinical complications. Clin Sci 87, 21–29.
Miller WM, Wilke CR and Blanch HW (1988) Transient responses of hybridoma cells to lactate and ammonia pulse and step changes in continuous culture. Bioproc Eng 3, 113.
Ozturk SS, Riley MR, and Palsson BO (1992) Effects of ammonia and lactate on hybridoma growth, metabolism and antibody production. Biotech Bioeng 39, 418–431.
Polgár L (1989) Mechanisms of protease action, CRC Press, Inc., Baton Rouge, FL.
Puchalski RB, Manoharan TH, Lathrop AL and Fahl WE (1991) Recombinant glutathione S-transferase (GST) expressing cells purified by flow cytometry on the basis of a GST-catalyzed intracellular conjugation of gutathione to monochlorobimane. Cytometry 12, 651–665.
Ranganathan S, Walsh ES, Godwin AK and Tew KD (1993) Cloning and characterization of human colon glyoxalase I. J Biol Chem 268, 5661–5667.
Ray M and Ray S (1987) Aminoacetone oxidase from goat liver. J. Biol Chem 262, 5974–5977.
Ray S and Ray M (1981) Isolation of a methylglyoxal synthase from goat liver. J Biol Chem 256, 6230–6234.
Rhee H, Murata K and Kimura A (1986) Purification and characterization of glyoxalase I from Pseudomonas putida. Biochem Biophys Res Commun 141, 993–999.
Rhee H, Murata K and Kimura A (1987) Molecular Cloning of the Pseudomonas putida gene in Escherichia coli. Biochem Biophys Res Commun 147 831–838.
Richard JP (1991) Kinetic parameters for the elimination reaction catalyzed by triosephosphate isomerase and an estimation of the reaction's physiological significance. Biochemistry 30, 4581–4585.
Shinohara M, Giardino I and Brownlee M (1996) Overexpression of glyoxalase I inhibits intracellular advanced glycation endproduct (AGE) formation. Diabetes 25, Supplement 2 126A.
Subramani S, Mulligan R and Berg P (1981) Expression of the mouse dihydrofolate reductase complementary deoxyribonucleic acid in SV40 vectors. Mol Cell Biol 1, 854–864.
Takahasi K (1977) Further studies on the reactions of phenylglyoxal and related reagents with proteins. J Biochem (Tokyo) 81, 403–414.
Thornalley PJ (1995) Advances in glyoxalase reasearch. Glyoxalase expression in malignancy, anti-proliferative effects of methylglyoxal, glyoxalase I inhibitor diesters and S-D-lactoylgluthathione, and methylglyoxal-modified protein binding and endocytosis by the advanced glycation endproduct receptor. Crit Rev Oncology & hematol 20, 99–128.
Thornalley PJ (1993) The glyoxalase system in health and disease. Mol Aspects Med 14, 287–371.
Thornalley PJ (1990) The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 269, 1–11.
Thornalley PJ (1988) Modification of the glyoxalase system in human red blood cells by glucose in vitro. Biochem J 254, 751–755.
Thornalley PJ, Edwards LG, Kang Y, Wyatt C, Davies N, Ladan MJ and Double J (1996) Antitumor activity of S-p-bromobenzylglutathione cyclopentyl diester in vitro and in vivo. Inhibition of glyoxalase I and the induction of apoptosis. Biochem Pharmacol, in press.
Van der Jagt DL, Robinson B, Taylor KT and Hunsaker LA (1992) Reduction of trioses by NADPH-dependent also-keto reductase. J Biol Chem 267, 4364–4369.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chaplen, F.W.R., Fahl, W.E. & Cameron, D.C. Effect of endogenous methylglyoxal on Chinese hamster ovary cells grown in culture. Cytotechnology 22, 33–42 (1996). https://doi.org/10.1007/BF00353922
Issue Date:
DOI: https://doi.org/10.1007/BF00353922