Summary
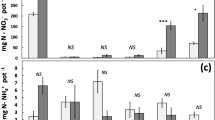
The effect of roots on microbial growth and N immobilization was investigated in a pot experiment with barley, Italian ryegrass, and white clover. We used a silty subsoil with a low soil organic matter content (0.16%C and 0.012%N), which allowed us to measure N immobilization as an increase in total soil organic N (planted versus unplanted). At sampling, the soil was easily removed from intact roots by gentle washing, with a negligible loss of root material. Plant growth and extra mineral N (in planted soil only) gave increased total counts (fluorescence microscopy) and viable counts (plate dilution) of bacteria, a higher proportion of larger cells, and increased viable counts as a percentage of total counts. Under monocots, 12–17% of the added fertilizer N was recovered as soil organic N. Although this N immobilization was attributed to microbial assimilation, less than 1/4 was actually recovered as microbial biomass N, as measured with the chloroform fumigation/N-extraction method or calculated from total bacterial counts. The white clover accumulated substantial amounts of N due to N2 fixation. However, microbial N immobilization represented only 3% of the total N accumulation, showing that the microorganisms obtained a smaller share of biologically fixed N2 than of the N applied as fertilizer. Extra additions of mineral N (monocots) enhanced microbial N assimilation, partly due to increased plant growth. The results also strongly indicated, however, that the microbial growth under monocots was N-limited in the latter part of the experiment and that fertilizer N had a direct effect on microbial growth. In the early phase of plant growth, N immobilization ranged from 33 to 58 mg N g-1 root C. This level of immobilization required a release of organic C into the soil representing a minimum of 60–100% of that found in intact roots.
Similar content being viewed by others
References
Bakken LR (1985) Separation and purification of bacteria from soil. Appl Environ Microbiol 49:1482–1487
Bakken LR (1988) Denitrification under different cultivated plants: effects of soil moisture tension, nitrate concentration, and photosynthetic activity. Biol Fertil Soils 6:271–278
Bakken LR, Olsen RA (1986) Dwarf cells in soil: A result of starvation of “normal” bacteria, or a separate population? In: Megusar F, Gantar M (eds) Perspectives in microbial ecology, Proc IV ISME. Slovene Society for Microbiology, Lubljana, pp 561–566
Barber DA, Martin JK (1976) The release of organic substances by cereal roots into soil. New Phytol 76:69–80
Bottner P, Sallih Z, Billes G (1988) Root activity and carbon metabolism in soils. Biol Fertil Soils 7:71–78
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Clarholm M (1985) Interactions of bacteria, protozoa and plants leading to mineralization of soil nitrogen. Soil Biol Biochem 17:181–187
Haider K, Mosier A, Heinemeyer O (1987) The effect of growing plants on denitrification at high nitrate concentrations. Soil Sci Soc Am J 51:97–102
Haller T, Stolp H (1985) Quantitative estimation of root exudation of maize plants. Plant and Soil 86:207–216
Hart PBS, Goh KM, Ludecke TE (1979) Nitrogen mineralisation in fallow and wheat soils under field and laboratory conditions. NZ J Agric Res 22:115–125
Helal HM, Sauerbeck D (1986) Effects of plant roots on carbon metabolism of soil microbial biomass. Z Pflanzenernähr Bodenkd 149:181–188
Henzell EF, Martin AE, Ross PJ, Haydock KP (1968) Isotopic studies on the uptake of nitrogen by pasture plants: IV. Uptake of nitrogen from labelled plant material by rhodes grass and siratro. Aust J Agric Res 19:65–77
Huntjens JLM (1971) The influence of living plants on mineralization and immobilization of nitrogen. Plant and Soil 35:77–94
Janzen HH, Bruinsma Y (1989) Methodology for the quantification of root and rhizosphere nitrogen dynamics by exposure of shoots to 15N-labelled ammonia. Soil Biol Biochem 21:189–196
Katznelson H (1965) Nature and importance of the rhizosphere. In: Baker KF, Snyder WC (eds) Ecology of soil-borne plant pathogens. University of California Press, Los Angeles, pp 187–207
Keith H, Oades JM, Martin JK (1986) Input of carbon to soil from wheat plants. Soil Biol Biochem 18:445–449
Marschner H (1986) Mineral nutrition of higher plants. Academic Press, London
McGill WB, Shields JA, Paul EA (1975) Relation between carbon and nitrogen turnover in soil organic fractions of microbial origin. Soil Biol Biochem 7:57–63
Merckx R, Dijkstra A, den Hartog A, van Veen JA (1987) Production of root-derived material and associated microbial growth in soil at different nutrient levels. Biol Fertil Soils 5:126–132
Nydahl F (1978) On the peroxodisulphate oxidation of total nitrogen in waters to nitrate. Water Res 12:1123–1130
Olsen RA, Bakken LR (1987) Viability of soil bacteria: Optimization of plate-counting technique and comparison between total counts and plate counts within different size groups. Microb Ecol 13:59–74
Pochon J (1954) Manuel technique d'analyse microbiologique du sol. Masson et Cie, Paris
Ritz K, Griffiths BS (1987) Effects of carbon and nitrate additions to soil upon leaching of nitrate, microbial predators and nitrogen uptake by plants. Plant and Soil 102:229–237
Rosswall T, Paustian K (1984) Cycling of nitrogen in modern agricultural systems. Plant and Soil 76:3–21
Schimel JP, Jackson LE, Firestone MK (1989) Spatial and temporal effects on plant-microbial competition for inorganic nitrogen in a California annual grassland. Soil Biol Biochem 21:1059–1066
Schnürer J, Rosswall T (1987) Mineralization of nitrogen from 15N labelled fungi, soil microbial biomass and roots and its uptake by barley plants. Plant and Soil 102:71–78
Turner SM, Newman EI, Campbell R (1985) Microbial population of ryegrass root surfaces: Influence of nitrogen and phosphorous supply. Soil Biol Biochem 17:711–715
Wang J, Bakken LR (1989) Nitrogen mineralization in rhizosphere and non-rhizosphere soil: Effect of the spatial distribution of N-rich and N-poor plant residues. In: Hansen JA, Henriksen K (eds) Nitrogen in organic wastes applied to soils. Academic Press, London, pp 81–97
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Breland, T.A., Bakken, L.R. Microbial growth and nitrogen immobilization in the root zone of barley (Hordeum vulgare L.), Italian ryegrass (Lolium multiflorum Lam.), and white clover (Trifolium repens L.). Biol Fertil Soils 12, 154–160 (1991). https://doi.org/10.1007/BF00341493
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00341493