Summary
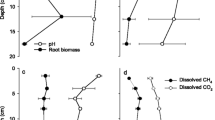
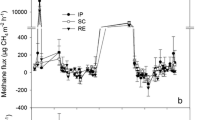
Dissolved O2 was depleted within the top 3.5-mm surface layer of flooded rice soil microcosms without plants. In planted microcosms, however, O2 was detectable down to at least 40 mm in depth. O2 concentrations in the uppermost soil layers of microcosms with rice plants were higher in the light than in the dark, indicating O2 production by photosynthesis. The CH4 emission rates were nearly identical for illuminated and for darkened microcosms, demonstrating that the photosynthetically produced O2 did not increase CH4 oxidation in the rhizosphere. In contrast, CH4 emission rates increased when the microcosms were incubated under an N2 atmosphere, indicating that transport of O2 from the atmosphere into the rhizosphere was important for CH4 oxidation. CH4 emission under air accounted for only 10%–20% of the cumulative CH4 production determined in cores taken from the microcosms. Apparently, 80%–90% of the CH4 produced was oxidized in the rhizosphere and thus was not emitted.
Similar content being viewed by others
References
Armstrong W (1979) Aeration in higher plants. Adv Bot Res 7:225–332
Berger U, Heyer J (1989) Untersuchungen zum Methankreislauf in der Saale. J Basic Microbiol 29:195–213
de Bont JAM, Lee KK, Bouldin DF (1978) Bacterial oxidation of methane in a rice paddy. Ecol Bull (Stockholm) 26:91–96
Born M, Dörr H, Levin I (1990) Methane consumption in aerated soils of the temperate zone. Tellus 42B:2–8
Conrad R, Rothfuss F (1991) Methane oxidation in the soil surface layer of a flooded rice field and the effect of ammonium. Biol Fertil Soils 12:28–32
Ehhalt DH (1988) How has the atmospheric concentration of CH4 changed? In: Rowland FS, Isaksen ISA (eds) The changing atmosphere. John Wiley and Sons, Chichester, pp 25–32
Frenzel P, Thebrath B, Conrad R (1990) Oxidation of methane in the oxic surface layer of a deep lake sediment (Lake Constance). FEMS Microbiol Ecol 73: 149–158
Gaynard TJ, Armstrong W (1987) Some aspects of internal plant aeration in amphibious habitats. In: Crawford RMM (ed) Plant life in aquatic and amphibious habitats. Blackwell Scientific, Oxford, pp 303–320
Helder W, Bakker JF (1985) Shipboard comparison of micro-and minielectrodes for measuring oxygen distribution in marine sediments. Limnol Oceanogr 30:1106–1109
Holzapfel-Pschorn A, Seiler W (1986) Methane emission during a cultivation period from an Italian rice paddy. J Geophys Res 91D:11803–11814
Holzapfel-Pschorn A, Conrad R, Seiler W (1985) Production, oxidation and emission of methane in rice paddies. FEMS Microbiol Ecol 31:343–351
Holzapfel-Pschorn A, Conrad R, Seiler W (1986) Effects of vegetation on the emission of methane from submerged paddy soil. Plant and Soil 92:223–233
Joshi MM, Hollis JP (1976) Interaction of Beggiatoa and rice plant: Detoxification and hydrogen sulfide in the rice rhizosphere. Science 195:179–180
Joshi MM, Ibrahim IKA, Hollis JP (1973) Oxygen release from rice seedlings. Physiol Plant 29:269–271
Joshi MM, Ibrahim IKA, Hollis JP (1975) Hydrogen sulfide: Effects on the physiology of rice plants and relation to straighthead disease. Phytopathology 65:1165–1170
Jørgensen BB, Revsbech NP (1983) Photosynthesis and struture of benthic microbial mats: Microelectrode and SEM studies of four cyanobacterial communities. Limnol Oceanogr 28:1075–1093
Keller M, Goreau TJ, Wofsy SC, Kaplan WA, McElroy MB (1983) Production of nitrous oxide and consumption of methane by forest soils. Geophys Res Lett 10:1156–1159
Khalil MA, Rasmussen RA (1989) Trends of atmospheric methane past present and future. In: Fantechi R, Ghazi A (eds) Carbon dioxide and other greenhouse gases: Climatic and associated impacts. Kluwer Academic Publishers, Dordrecht, pp 91–100
Khalil MAK, Rasmussen RA, Wang MX, Ren L (1990) Emissions of trace gases from Chinese rice fields and biogas generators: Methane, dinitrogen oxide, carbon monoxide, carbon dioxide chlorocarbons, and hydrocarbons. Chemosphere 20:207–226
King GM (1990) Regulation by light of methane emissions from a wetland. Nature 345:513–515
Kuivila KM, Murray JW, Devol AH, Lidstrom ME, Reimers CE (1988) Methane cycling in the sediments of Lake Washington. Limnol Oceanogr 33:571–581
Matthews E, Fung I (1987) Methane emission from natural wetlands: Global distribution, area, and environmental characteristics of sources. Global Biochem Cycles 1:61–86
Mayer HP, Conrad R (1990) Factors influencing the population of methanogenic bacteria and the initiation of methane production upon flooding of paddy soil. FEMS Microbiol Ecol 73:103–112
Nouchi I, Mariko S, Aoki K (1990) Mechanism of methan transport from the rhizosphere to the atmosphere through rice plants. Plant Physiol 94:59–66
Revsbech NP (1989) An oxygen microsensor with a guard cathode. Limnol Oceanogr 34: 474–478
Revsbech NP, Ward DM (1983) Oxygen microelectrode that is insensitive to medium chemical composition: Use in an acid microbial mat dominated by Cyanidium caldarium. Appl Environ Microbiol 45:755–759
Revsbech NP, Jørgensen BB, Blackburn TH, Cohen J (1983) Microelectrode studies of the photosynthesis of O2, H2S, and pH profiles of a microbial mat. Limnol Oceanogr 28:1062–1074
Schütz H, Seiler W, Conrad R (1989) Processes involved in formation and emission of methane in rice paddies. Biogeochemistry 733–53
Schütz H, Seiler W, Conrad R (1990a) Influence of soil temperature on methane emission from rice paddy fields. Biogeochemistry 11:77–95
Schütz H, Seiler W, Rennenberg H (1990b) Soil and land use related sources and sinks of methane (CH4) in the context of the global methane budget. In: Bouwman AF (ed) Soils and the greenhouse effect, vol 12, John Wiley & Sons, Chichester, pp 269–285
Seiler W, Conrad R, Scharffe D (1984) Field studies of methane emission from termite nests into the atmosphere and measurements of methane uptake by soils. J Atmos Chem 1:171–186
Sweerts J-PRA (1990) Oxygen consumption processes, mineralization and nitrogen cycling at the sediment-water interface of north temperate lakes. Ph D thesis, Univ Groningen
Whalen SC, Reeburgh WS, Sandbeck KA (1990) Rapid methane oxidation in a landfill cover soil. Appl Environ Microbiol 56:3405–3411
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Frenzel, P., Rothfuss, F. & Conrad, R. Oxygen profiles and methane turnover in a flooded rice microcosm. Biol Fertil Soils 14, 84–89 (1992). https://doi.org/10.1007/BF00336255
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00336255