Abstract
The enemy release hypothesis states that introduced plants have a competitive advantage due to their release from co-evolved natural enemies (i.e., herbivores and pathogens), which allows them to spread rapidly in new environments. This hypothesis has received mixed support to date, but previous studies have rarely examined the herbivore community, plant damage, and performance simultaneously and largely ignored below-ground herbivores. We tested for enemy release by conducting large scale field surveys of insect diversity and abundance in both the native (United Kingdom) and introduced (New Zealand) ranges of three dock (Rumex, Polygonaceae) species: R. conglomeratus Murray (clustered dock), R. crispus L. (curly dock) and R. obtusifolius L. (broad-leaved dock). We captured both above- and below-ground insect herbivores, measured herbivore damage, and plant biomass as an indicator for performance. In the introduced range, Rumex plants had a lower diversity of insect herbivores, all insect specialists present in the native range were absent and plants had lower levels of herbivore damage on both roots and leaves. Despite this, only R. crispus had greater fresh weight in the introduced range compared to the native range. This suggests that enemy release, particularly from below-ground herbivores, could be a driver for the success of R. crispus plants in New Zealand, but not for R. conglomeratus and R. obtusifolius.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The success of introduced plants is often credited to their escape from co-evolved natural enemies (Keane and Crawley 2002; Lake and Leishman 2004; Lambert and Casagrande 2006; Mukherjee et al. 2012). If plants experience enemy escape, then the Enemy Release Hypothesis (ERH) proposes that if plants are limited by herbivores in their native range, loss of these natural enemies in the introduced range will increase their survival, biomass and fecundity (Williamson and Fitter 1996; Simberloff and Von Holle 1999; Richardson et al. 2000; Maron and Vila 2001; Kolar and Lodge 2001; Keane and Crawley 2002; Bruno et al. 2003; Daehler 2003; Duncan et al. 2003; Keane and Crawley 2002; Huang et al. 2018). Multiple studies, including a meta-analysis (Liu and Stiling 2006), have supported the ERH (Bigger and Marvier 1998; Colautti et al. 2004; Kater 2016; Torchin et al. 2001). In their native range, plants are exposed to pressures from both specialist and generalist herbivores, but specialist herbivores that have coevolved with the plant host rarely occur outside the native range of their host plant (Orians and Ward, 2010). As a result, plant species in the introduced range may have reduced herbivore pressure with mostly polyphagous generalist herbivores, while in their native range they may have both generalist and specialist natural enemies (Frenzel and Brandl 2003; Orians and Ward 2010; Van Lenteren et al. 2003). Studies comparing herbivore communities and the level of herbivory between the native and introduced ranges have found that plants in the introduced range often experience a lower overall diversity of natural enemies, a shift from specialists to generalist herbivores, and reduced herbivory (Colautti et al. 2004; Hinz and Schwarzlaender 2004; Willis et al. 2000), all indicative of enemy escape and possibly leading to enemy release. The success of biocontrol agents underscores the potential for natural enemies to control the population dynamics of their host plant, which can also be taken as evidence for the ERH (DeLoach 1995).
Most studies addressing the ERH that have undertaken field surveys have only examined one or two plant species (Meijer et al. 2016) and typically only look at above-ground natural enemies (Huang et al. 2018). The fact that below-ground insect herbivores have received so little attention is unfortunate, as they are pervasive in most terrestrial ecosystems and play a crucial role in moderating the spread and abundance of plants (Johnson and Rasmann 2015; van Dam 2009; Van Der Putten et al. 2009). Shoot herbivory can lead to a reduction of photosynthetic tissue (Hambäck 2001) and reproductive output (Karban and Strauss 1993; Zangerl et al. 2002). Root herbivory can reduce water and nutrient uptake and disrupt their transportation within the plant (Blossey and Hunt-Joshi 2003; Zvereva and Kozlov 2012). Furthermore, shoot and root herbivory may interactively influence plant fitness (Johnson et al. 2016; Maron 1998).
In this study, we tested for both enemy escape and enemy release between the native (United Kingdom) and introduced (New Zealand) ranges of three species of Rumex (R. conglomeratus Murray, R. crispus L. and R. obtusifolius L.). Here, enemy escape is defined as the absence of specialists in the introduced range, whereas enemy release also implies that plants would have evolved as a results of this reduced specialist pressure. Each of the three plant species are native to Eurasia, occupy a wide range of natural and cultivated habitats across the world (Kubát 1990) and are considered problematic weeds in both the native and introduced environments (Bond et al. 2007). The three Rumex species serve as a good model for testing the ERH across a suite of related species because they: (i) have multiple specialist herbivores in the native range (Martinková and Honěk 2004); (ii) occupy similar habitats and often co-occur in both the native and introduced ranges; (iii) were introduced to New Zealand almost two centuries ago, which should have given them sufficient time to acquire natural enemies in their new environment (Atwood and Meyerson 2011); and (iv) were accidental introductions and thus not exposed to deliberate plant breeding that might influence plant traits such as size.
There are at least 32 species of insect herbivores that feed on R. crispus and R. obtusifolius in the native range (Cavers and Harper 1964). This includes several herbivores that are sufficiently specialized on, and damaging to Rumex, that they have been considered as potential biological control agents: the shoot herbivores Gastrophysa viridula (De Geer) (Coleoptera, Chrysomelidae) (Martinková and Honěk 2004), Hypera rumicis L. (Coleoptera, Curculionidae), species of Apion weevils (Grossrieder and Keary 2004) and the root herbivore Pyropteron chrysidiformis (Esper) (Lepidoptera, Sesiidae) (Hahn et al. 2016). In particular, Gastrophysa viridula can reduce Rumex species seed production (Bentley et al. 1980; DeGregorio et al. 1992), regeneration (DeGregorio et al. 1992), leaf and shoot growth (Cottam et al. 1986) and alter Rumex cover (Kohout and Kohoutova 1994; Kohout 1994). The larvae of clearwing moths, Pyropteron spp., can increase root decay and reduce the number of rosettes in R. obtusifolius (Hahn et al. 2016).
Here we tested three predictions of the ERH: (i) insect herbivore communities in the native range will be more diverse than in the introduced range; (ii) the introduced range contains fewer specialist herbivores than the native range; (iii) Rumex in the introduced range experience lower levels of herbivory and are larger than their counterparts in the native range.
Materials and methods
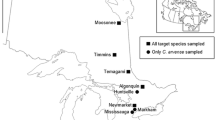
To quantify invertebrate herbivore abundance, particularly of specialist natural enemies, as well as leaf and root herbivory, field surveys were conducted in June and July of 2017 in the United Kingdom (UK, native range) and December 2017 and January 2018 in New Zealand (NZ, introduced range). These sampling periods represent the summer in both ranges, when plants were large and not yet senescent. We sampled across England and Scotland in the UK and across the South Island of NZ to encompass a similar array of climatic conditions in both ranges across a large latitudinal gradient as both climate and latitude can influence the strength of enemy release (Allen et al. 2017). For R. crispus and R. obtusifolius, 16 populations were surveyed from each range, but for R. conglomeratus only 12 populations were surveyed in the UK, as only four populations could be located in Scotland. The exact coordinates of the sampling locations can be found in the supplementary material.
Insect and leaf collection in the native and introduced ranges
In each range, insect and leaf samples were collected from 10 individual plants per population (Fig. 1). Although all three Rumex species are widespread in both the UK and NZ, we targeted populations that had at least 30 individuals growing in close proximity. In both the UK and NZ, populations were sampled across a range of temperature and precipitation environments which largely overlapped across the two ranges (Carlin 2022). All populations were located below 300 m elevation, at least 1 km away from the coast in open habitat and at least 3.5 km away from other surveyed conspecific populations (mean = 38 km, range = 3.5 to 120.8 km). For the most part, each location only contained a single Rumex species, but a few sites in both the native and introduced range contained two species. Since multiple Rumex species were often found growing in close proximity to each other, prior to collection, each plant was examined to confirm species identity and exclude obvious hybrids based on morphological traits. In both ranges, we only collected from plants that were either flowering or beginning to set seed, to standardize plant life stage and available herbivore habitat.
To collect insect herbivores, each Rumex plant was cut at ground level, placed in a bag and vigorously shaken to cause insects to drop into the bag. The plant was also inspected for insects that did not fall after shaking. In the UK, stem burrowing weevils, such as Apion violaceum Kirk can be found in their larval state on Rumex from mid-May to mid-June (Freese 1995). This means that sampling in the native range took place after they emerge into adulthood, which is why stems were not inspected for weevil larvae but adults were nevertheless collected on the plants. All insects were stored in 70% ethanol for later identification. This technique is similar to the beat sheet method which is fast, has low variability (Turnipseed 1974) and is effective for counting mobile insects (Deutscher et al. 2003). The roots of each plant were also collected to check for any root feeders inside the central tap root and were scored as damaged by disease (evidence of root rot) and/or herbivory (present/absent).
The five largest leaves from each surveyed plant were collected and photographed to determine the percentage of leaf area damaged by herbivores. The photos were analysed using the image processing program BioLeaf (Machado et al. 2016). To test for differences in performance, we measured fresh biomass by cleaning and weighing the above- and below-ground parts of the collected plants on the same day as collection. Biomass is correlated with seed production in short-lived herbaceous species like Rumex (Pino et al. 2002; Grime et al. 2007).
All herbivorous insect specimens were separated into morphospecies and a representative from each of these groups was identified to order and family (where possible) (Coleoptera: Brentidae, Curculionidae, Elateridae, Nitidulidae, Scarabaeidae; Diptera: Tipulidae; Forficulidae; Hemiptera: Aphidae, Cercopidae, Coreidae, Miridae, Pentatomidae, Psillidae; Lepidoptera: Sesiidae; and Thysanoptera). Known Rumex-specialist insects and any insects found in both the native and introduced ranges were identified to the species level: Gastrophysa viridula (De Geer), Hypera rumicis (L), Coreus marginatus (L), Pyropteron chrysidiforme (Esper), Philaenus spumarius (L) and Closterotomus norwegicus (Gmelin). We chose to group insects by morphospecies because they are accurate surrogates for estimates of species numbers (Oliver and Beattie 1996) and the large number of individuals collected meant identifying each to species was not feasible. Morphospecies were classified by feeding mode as chewers or sap-suckers. Chewing insects cause damage that can be visually quantified on leaves and typically include the orders Coleoptera (adults and larvae), Lepidoptera (larvae), Symphyta (sawfly larvae), Orthoptera and Phasmatodea (Elliott et al. 1998). Phloem feeders can remove as much biomass as chewers, but damage from sap-sucking insects is less obvious and more difficult to quantify (Leigh 1997). Sap-sucking insects belong to the orders Hemiptera and Thysanoptera (Elliott et al. 1998).
Statistical analyses
To test whether insect communities differed between the native and introduced ranges, we compared insect abundance across common orders. We tested whether the abundance of insects in each order in NZ was significantly different from what we would expect based on observations in the UK using a chi-squared test. This analysis included four insect orders (Thysanoptera, Lepidoptera, Hemiptera, Coleoptera) and “Others”, which due to the very low number of insect individuals (< 20 per order), included all other orders.
Differences in the diversity of insect herbivore morphospecies found on Rumex plants between the native (UK) and introduced (NZ) ranges were calculated using an unpaired t-test of the Shannon index entropy at the population level \(\left( {H^{\prime}} \right) = - \mathop \sum \limits_{i = 1}^{s} p_{i} \ln p_{i}\), where pi is the proportion of individuals of species i divided by the total number of individuals across all species. The number of species is s. Morphospecies richness (the number of insect morphospecies) and total abundance of chewing and sucking insects per plant was modelled using generalized linear mixed effects models with a Poisson distribution. Each model had fixed effects of range (UK or NZ) and total plant fresh weight, which was centred and scaled (Schielzeth 2010), and population was included as a random effect.
To assess whether Rumex species herbivore damage differed between the native and introduced ranges, we compared rates of damage for both above- and below-ground plant parts across ranges. To analyse the proportion of roots diseased or damaged within a population we used generalized linear models with a binomial distribution and range (UK or NZ) as the predictor. For the analyses of the percentage of leaf area lost to herbivory and of total plant fresh weight, we used linear mixed effects models with a Gaussian distribution, where range (UK or NZ) was included as a fixed effect and population as a random effect. Percentage of leaf area lost was calculated as the average across five leaves per plant.
To determine the significance of the range effect, a Wald Z test or a Wald Chi-squared test were used. Each Rumex species was modelled separately. Residual plots and diagnostics were checked for all analyses to ensure the validity of the model assumptions. All statistical analyses were performed in R (R Core Team 2019) and mixed models were run using ‘lme4’ R package v.1.1–19 (Bates et al. 2015).
Results
Abundance and diversity of insect herbivores between native and introduced ranges
The abundance of insects across orders in NZ was significantly different from what would be expected based on the abundance of insects across orders in the UK: R. conglomeratus (χ2 = 1705, df = 4, p < 0.001), R. crispus (χ2 = 2544, df = 4, p < 0.001) and R. obtusifolius (χ2 = 3606, df = 4, p < 0.001). In the introduced range, all three Rumex species had fewer Coleoptera and Thysanoptera individuals (Fig. 2). In both ranges, Hemiptera individuals were highly abundant and Lepidoptera individuals were uncommon (Fig. 2).
There was a significantly greater Shannon entropy of herbivorous insect morphospecies in the native range for R. conglomeratus (p < 0.001) and R. obtusifolius (p = 0.032), but not for R. crispus (p = 0.796) (Fig. 3). Rumex conglomeratus, R. crispus and R. obtusifolius all had significantly greater abundance of chewing insects in the native range than in the introduced range (p ≤ 0.004, Fig. 4). However, no differences between ranges in sap-sucking insect abundance were detected for any Rumex species (p ≥ 0.17) (Fig. 4).
In the native range, the leaf specialist Gastrophysa viridula was found on 3% of R. conglomeratus (mean ± 1SE 0.09 ± 0.06 insects per plant), 10% of R. crispus (0.94 ± 0.32) and 16% of R. obtusifolius (1.26 ± 0.45) and the root specialist Pyropteron chrysidiforme was found on 2% of R. conglomeratus (mean ± 1SE insects per plant 0.02 ± 0.01), and 6% of R. crispus and R. obtusifolius (0.06 ± 0.02). No specialist insect herbivores were found in the introduced range. However, two generalist hemipteran species native to Europe, Philaenus spumarius and Closterotomus norwegicus, were present in large numbers on plants in both ranges.
Root condition and leaf herbivore damage
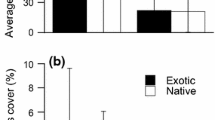
The presence of root herbivory was significantly higher in Rumex plants in the native range than in the introduced range for all three study species (Fig. 5a). However, only R. conglomeratus plants had a significantly higher incidence of root disease in the native range (p < 0.001; Fig. 5a). Leaf damage in the native range was rarely more than 4%, but was on average six times greater (mean ± SE 3.43 ± 0.24) than in the introduced range (0.58 ± 0.05) for all three Rumex study species (p < 0.001; Fig. 5b).
Plant biomass
In the introduced range, R. crispus plants had significantly (p = 0.04) higher total fresh weight than in the native range. The other two Rumex species showed no significant differences in the total fresh weight between ranges (Fig. 6).
Discussion
We provide evidence of enemy escape as a result of fewer herbivorous insects and indicated by lower damage in the introduced range for all three Rumex species. Even though damage was lower in the introduced range (NZ), only R. crispus showed evidence for enemy release, having a higher biomass in NZ. Two Rumex species (R. conglomeratus and R. obtusifolius) had a greater diversity of insect morphospecies in the native range (UK), but no difference between ranges was observed for R. crispus. All three Rumex species had a greater abundance of chewing insect herbivores, as well as greater leaf and root damage in the native range, as opposed to the introduced range. Specialist insect herbivores, although found frequently on plants in the native range, were absent from the introduced range.
At a family level, there was a greater diversity and abundance of chewing insect herbivores on Rumex plants in the native range than in the introduced range, which may reflect the fact that the native range hosts a greater variety of chewing insect families than the introduced range. For example, in the UK there are 103 Coleoptera families (UK Natural History Museum 2020), whereas in New Zealand, there are only 82 Coleoptera families (Leschen et al. 2003). However, among sap-sucking insects the same six Hemiptera families were found on plants from both the native and introduced range and Hemipteran abundance was similar between ranges, despite greater diversity in the UK. Alternatively, lower diversity and abundance in the families of insect herbivores could be a result of the lack of co-evolved herbivores in the introduced range, as predicted for enemy escape (Keane and Crawley 2002).
For all three Rumex study species, specialist herbivores like Gastrophysa viridula and Pyropteron chrysidiforme, although fairly common in the native range, were absent from the introduced range. Thus, it appears that introduced Rumex populations have indeed escaped from specialist herbivores, as have other species (Kwon 2008; Kwon et al. 2006; Park et al. 2008), such as tall goldenrod, Solidago altissima L. (Asteraceae) (Uesugi and Kessler 2016), and creeping thistle, Cirsium arvense (L.) Scop. (Cripps et al. 2011, 2010). Instead, insect herbivores feeding on the three Rumex species in the introduced range have been recorded as generalist feeders in the native range (Cavers and Harper 1964), consistent with the predictions for arthropods colonizing introduced plants (Fraser and Lawton 1994) and other comparative studies (Goeden 1974; Jobin et al. 1996; Memmott et al. 2000). Introduced plants can also be colonised by native insect species, which can thus act as potential biocontrol agents, as was the case for R. obtusifolius in Korea, where it encountered Gastrophysa atrocyanea (Motschulsky) (Coleoptera, Chrysomelidae), Ostrinia palustralis memnialis (Walker) (Lepidoptera: Crambidae) and Allantus luctifer (Smith) (Hymenoptera: Tenthredinidae). Alternatively, specialist herbivores can sometimes be introduced either at the same time as the host (Canavan et al. 2019) or at a later date and the longer the residence time, the more likely this is to happen by chance (Hawkes 2007; Schultheis et al. 2015). For example, the parsnip webworm (Depressaria pastinacella Goeze (Depressariidae)) re-associated with wild parsnip (Pastinaca sativa L. (Apiaceae)) roughly 150 years after the introduction of wild parsnip to New Zealand (Zangerl et al. 2008). Although no specialist Rumex herbivores have been introduced from their native range, two generalist sap-sucking insect herbivores native to the UK, Philaenus spumarius L. (Hemiptera, Aphrophoridae) and Closterotomus norwegicus (Gmelin) (Hemiptera, Miridae), were frequently present on Rumex plants in both ranges. As they were found on 18–24% of surveyed plants and have been present in NZ since at least the 1960s (Archibald et al. 1979; Myers and China 1928), it is possible that these two species could have limited enemy release for R. conglomeratus and R. obtusifolius. Of note is the fact that New Zealand has two native Rumex species (R. flexuosus Sol. and R. neglectus Kirk), both of which are endemic and of cultural importance for the Māori people. This would make it unlikely that specialists will be introduced to New Zealand for the biocontrol of invasive Rumex species, as there would be a high chance that the native species will also be affected.
Rumex plants in the native range had significantly higher herbivore damage on both roots and leaves than plants in the introduced range, as predicted by the ERH and found in other studies (Adams et al. 2009; Castells et al. 2013; Cripps et al. 2006; DeWalt et al. 2004; Memmott et al. 2000; Veselkin et al. 2019; Vilà et al. 2005 but see Williams et al. 2010), including in New Zealand (Fenner and Lee 2001; Lieurance and Cipollini 2012, but see Cripps et al. 2010). Leaf herbivory in our system was low in both ranges, which could mean herbivory had only a modest effect on plant performance. Other studies that found low levels of leaf damage (< 10%), similarly did not find support for enemy release in the introduced range (Adams et al. 2009; Kater 2016; Lieurance and Cipollini 2012). Gastrophysa viridula has been shown to significantly reduce the performance of Rumex (Moore et al. 2003), although this only occurs at experimental densities of at least two adult beetles and their offspring per plant (Bentley and Whittaker 1979), whereas under natural conditions we found an average of 0.83 individuals (adult and larvae) per plant. This suggests that most of the leaf damage was not caused by chewing insects, but possibly by snails and slugs, which can be found frequently on Rumex plants in both the native and introduced ranges (Costan C. A., pers. obs.). Mammalian herbivores or anthropogenic disturbances can also greatly affect the development and growth of Rumex plants, but in this study, we excluded highly disturbed or heavily grazed sites. Thus, it is likely that for the plants that we surveyed, insect herbivory played a prominent role.
Together these results suggest enemy escape in the introduced range, but there is little evidence of a performance increase, and therefore enemy release, as a result of this loss of natural enemies. Since fecundity is likely highly corelated to biomass in Rumex (Pino et al. 2002; Bufford and Hulme 2021), an increase in biomass would be a good indication of success. In the introduced range, R. crispus had a significantly greater fresh weight than in the native range, possibly as a result of lower root herbivory compared to R. crispus plants in the native range. Both Rumex conglomeratus and R. obtusifolius had lower root herbivory than R. crispus and tend to have multiple tap roots, while R. crispus plants generally have one main tap root with a larger diameter (Cavers and Harper 1964). This makes R. crispus a more favourable target for attack by larvae of the specialist clearwing moth, Pyropteron chrysidiforme, which needs a sufficiently large root diameter to survive (Spafford et al. 2008). Consistent with this, a closely related insect species Pyropteron doryliformis (Ochsenheimer), which was introduced as a biocontrol agent in Australia, established on R. crispus plants but not on R. obtusifolius and R. conglomeratus (Morley et al. 2008). Plant biomass was similar for R. conglomeratus and R. obtusifolius between ranges, suggesting that the differences in herbivory had modest effects on performance and that escape from specialist herbivores does not always result in an increased plant performance (Chun et al. 2010). This provides at best mixed evidence for the ERH and that evidence is primarily for effects of below-ground herbivores, which are less often examined in ERH studies.
Conclusion
This work provides multi-species evidence that invasive plant species can escape from specialist insect herbivores in their introduced range and, although hosting a different insect herbivore community, experience greatly reduced herbivory compared to their counterparts in their native range. Herbivore damage in both ranges was low, however, and no significant difference in plant biomass was observed between the native and introduced ranges for R. conglomeratus and R. obtusifolius, suggesting that enemy escape is not a main driver of the success of these two Rumex species in New Zealand. For R. crispus, however, release from root herbivory in the native range may explain the significantly greater fresh weight of R. crispus plants in the introduced range, which could indicate support for the enemy release hypothesis for this species in New Zealand and emphasizes the importance of considering below-ground interactions.
Data availability
If the manuscript is accepted for publication, the data will be archived in Figshare.
Code availability
The code generated during the study is available upon reasonable request.
References
Adams JM, Fang W, Callaway RM, Cipollini D, Newell E, Acer T, TRAIN (2009) A cross-continental test of the enemy release hypothesis: leaf herbivory on Acer platanoides (L.) is three times lower in North America than in its native Europe. Biol Invasions 11:1005–1016. https://doi.org/10.1007/s10530-008-9312-4
Allen WJ, Meyerson LA, Cummings D, Anderson J, Bhattarai GP, Cronin JT (2017) Biogeography of a plant invasion: drivers of latitudinal variation in enemy release. Glob Ecol Biogeogr 26(4):435–446. https://doi.org/10.1111/geb.12550
Archibald RD, Cox JM, Deitz LL (1979) New records of plant pests in New Zealand. N Z J Agric Res 22(1):201–207. https://doi.org/10.1080/00288233.1979.10420862
Atwood J, Meyerson L (2011) Beyond EICA: understanding post-establishment evolution requires a broader evaluation of potential selection pressures. NeoBiota 10:7–25. https://doi.org/10.3897/neobiota.10.954
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Bentley S, Whittaker J (1979) Effects of grazing by a chrysomelid beetle, Gastrophysa viridula, on competition between Rumex obtusifolius and Rumex crispus. J Ecol 67(1):79–90. https://doi.org/10.2307/2259338
Bentley S, Whittaker JB, Malloch JC (1980) Field experiments on the effects of grazing by a chrysomelid beetle (Gastrophysa-Viridula) on seed production and quality in Rumex obtusifolius and Rumex crispus. J Ecol 68(2):671–674
Bigger DS, Marvier MA (1998) How different would a world without herbivory be?: a search for generality in ecology. Integr Biol Issues News Rev 1(2):60–67. https://doi.org/10.1002/(sici)1520-6602(1998)1:2%3c60::aid-inbi4%3e3.0.co;2-z
Blossey B, Hunt-Joshi TR (2003) Belowground herbivory by insects: influence on plants and aboveground herbivores. Annu Rev Entomol 48(1):521–547. https://doi.org/10.1146/annurev.ento.48.091801.112700
Bond W, Davies G, Turner R (2007) The biology and non-chemical control of broad-leaved dock (Rumex obtusifolius L.) and curled dock (R. crispus L.). HDRA, Coventry, UK., (November), 1–15
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18(3):119–125. https://doi.org/10.1016/S0169-5347(02)00045-9
Bufford JL, Hulme PE (2021) Seed size–number trade-offs are absent in the introduced range for three congeneric plant invaders. J Ecol 109(11):3849–3860. https://doi.org/10.1111/1365-2745.13761
Canavan K, Paterson ID, Hill MP, Dudley TL (2019) Testing the enemy release hypothesis on tall-statured grasses in South Africa, using Arundo donax, Phragmites australis, and Phragmites mauritianus as models. Bull Entomol Res 109(3):287–299. https://doi.org/10.1017/S0007485318000627
Carlin TF (2022) Assessing range limits and niche shifts in invasive weeds: a thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy at Lincoln University, Lincoon Canterbury NZ
Castells E, Morante M, Blanco-Moreno JM, Sans FX, Vilatersana R, Blasco-Moreno A (2013) Reduced seed predation after invasion supports enemy release in a broad biogeographical survey. Oecologia 173(4):1397–1409. https://doi.org/10.1007/s00442-013-2718-4
Cavers PB, Harper JL (1964) Biological flora of the British Isles. Rumex obtusifolius L. and R. crispus L. J Ecol 52:737–766. https://doi.org/10.2307/2257859
Chun YJ, van Kleunen M, Dawson W (2010) The role of enemy release, tolerance and resistance in plant invasions: linking damage to performance. Ecol Lett 13:937–946. https://doi.org/10.1111/j.1461-0248.2010.01498.x
Colautti RI, Ricciardi A, Grigorovich IA, MacIsaac HJ (2004) Is invasion success explained by the enemy release hypothesis? Ecol Lett 7:721–733. https://doi.org/10.1111/j.1461-0248.2004.00616.x
Cottam DA, Whittaker JB, Malloch AJC (1986) The effects of chrysomelid beetle grazing and plant competition on the growth of Rumex obtusifolius. Oecologia 70:452–456. https://doi.org/10.1007/BF00379511
Cripps MG, Bourdôt GW, Saville DJ, Hinz HL, Fowler SV, Edwards GR (2011) Influence of insects and fungal pathogens on individual and population parameters of Cirsium arvense in its native and introduced ranges. Biol Invasions 13(12):2739–2754. https://doi.org/10.1007/s10530-011-9944-7
Cripps MG, Edwards GR, Bourdôt GW, Saville DJ, Hinz HL, Fowler SV (2010) Enemy release does not increase performance of Cirsium arvense in New Zealand. Plant Ecol 209(1):123–134. https://doi.org/10.1007/s11258-010-9728-7
Cripps MG, Schwarzländer M, McKenney JL, Hinz HL, Price WJ (2006) Biogeographical comparison of the arthropod herbivore communities associated with Lepidium draba in its native, expanded and introduced ranges. J Biogeogr 33(12):2107–2119. https://doi.org/10.1111/j.1365-2699.2006.01560.x
Daehler CC (2003) Performance comparisons of co-occurring native and alien invasive plants: implications for conservation and restoration. Annu Rev Ecol Evol Syst 34:183–211. https://doi.org/10.1146/annurev.ecolsys.34.011802.132403
DeGregorio RE, Ashley RA, Streams FA, Schaefer CW, Adams RG (1992) Biocontrol potential of Hypera rumicis (l.) (Coleoptera: Curculionidae) on curly dock (Rumex crispus l.). J Sustain Agric 2(1):7–24. https://doi.org/10.1300/J064v02n01_03
DeLoach CJ (1995) Progress and problems in introductory biological control of native weeds in the United States. In: Delfosse ES, Scott RR (eds) Proceedings of the eighth international symposium on biological control of weeds, CSIRO Publishing, Collingwood, 111–112
Deutscher S, Dillon M, Mckinnon C, Mansfield S, Staines T, Lawrence L (2003) Giving insects a good beating. Aust Cottongrower 24(January2003):24
DeWalt SJ, Denslow JS, Ickes K (2004) Natural-enemy release facilitates habitat expansion of the invasive tropical shrub Clidemia hirta. Ecology 85(2):471–483. https://doi.org/10.1890/02-0728
Duncan RP, Blackburn TM, Sol D (2003) The ecology for bird introductions. Annu Rev Ecol Evol Syst 34:71–98. https://doi.org/10.1146/annurev.ecolsys.34.011802.132353
Elliott HJ, Ohmart CP, Wylie FR (1998) Insect pests of Australian forests: ecology and management. Inkata Press, Melbourne
Fenner M, Lee WG (2001) Lack of pre-dispersal seed predators in introduced Asteraceae in New Zealand. New Zealand J Ecol 25:95–99
Fraser SM, Lawton JH (1994) Host range expansion by British moths onto introduced conifers. Ecol Entomol 19(2):127–137. https://doi.org/10.1111/j.1365-2311.1994.tb00402.x
Freese G (1995) Structural refuges in two stem-boring weevils on Rumex crispus. Ecol Entomol 20(4):351–358. https://doi.org/10.1111/j.1365-2311.1995.tb00467.x
Frenzel M, Brandl R (2003) Diversity and abundance patterns of phytophagous insect communities on alien and native host plants in the Brassicaceae. Ecography 26(6):723–730. https://doi.org/10.1111/j.0906-7590.2003.03649.x
Goeden RD (1974) Comparative survey of the phytophagous insect faunas of Italian thistle, Carduus pycnocephalus, in Southern California and Southern Europe relative to biological weed control. Environ Entomol 3(3):464–474. https://doi.org/10.1093/ee/3.3.464
Grime JP, Hodgson JG, Hunt R (2007) Comparative plant ecology: a functional approach to common British species. Springer
Grossrieder M, Keary IP (2004) The potential for the biological control of Rumex obtusifolius and Rumex crispus using insects in organic farming, with particular reference to Switzerland. Biocontrol News Inf 25(3):65–79
Hahn MA, Schaffner U, Häfliger P, Lüscher A (2016) Establishment and early impact of the native biological control candidate Pyropteron chrysidiforme on the native weed Rumex obtusifolius in Europe. Biocontrol 61(2):221–232. https://doi.org/10.1007/s10526-016-9715-6
Hambäck PA (2001) Direct and indirect effects of herbivory: feeding by spittlebugs affects pollinator visitation rates and seedset of Rudbeckia hirta. Ecoscience 8(1):45–50. https://doi.org/10.1080/11956860.2001.11682629
Hawkes CV (2007) Are invaders moving targets? The generality and persistence of advantages in size, reproduction, and enemy release in invasive plant species with time since introduction. Am Nat 170:832–843. https://doi.org/10.1086/522842
Hinz HL, Schwarzlaender M (2004) Comparing invasive plants from their native and exotic range: what can we learn for biological control? 1. Weed Technol 18(sp1):1533–1541. https://doi.org/10.1614/0890-037x(2004)018[1533:cipftn]2.0.co;2
Huang W, Siemann E, Ding J (2018) Eco-evolutionary dynamics of above- and belowground herbivores and invasive plants. Above-Ground Commun Ecol 234:271–291. https://doi.org/10.1007/978-3-319-91614-9_12
Jobin A, Schaffner U, Nentwig W (1996) The structure of the phytophagous insect fauna on the introduced weed Solidago altissima in Switzerland. Entomol Exp Appl 79(1):33–42. https://doi.org/10.1111/j.1570-7458.1996.tb00806.x
Johnson SN, Erb M, Hartley SE (2016) Roots under attack: contrasting plant responses to below- and aboveground insect herbivory. New Phytol 210(2):413–418. https://doi.org/10.1111/nph.13807
Johnson SN, Rasmann S (2015) Root-feeding insects and their interactions with organisms in the rhizosphere. Annu Rev Entomol 60(1):517–535. https://doi.org/10.1146/annurev-ento-010814-020608
Kater SS (2016) Can enemy release explain the invasion success of the diploid Leucanthemum vulgare in North America? Biol Invasions 18:2077–2091. https://doi.org/10.1007/s10530-016-1152-z
Karban R, Strauss SY (1993) Effects of herbivores on growth and reproduction of their perennial host Erigeron glaucus. Ecology 74(1):39–46. https://doi.org/10.2307/1939499
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17(4):164–170. https://doi.org/10.1016/S0169-5347(02)02499-0
Kohout V (1994) The weevil (Apion miniatum Germar)—A biological regulator of distribution of broad-leaved docks. Ochrana Rostlin 30:79–81
Kohout V, Kohoutova SN (1994) Possibilities of utilization of species Apion miniatum Germar in biological control of genus Rumex. Zeitschrift Fu¨r Pflanzenkrankheiten Und Pflanzenschutz 14, 217–220
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Ecol Evol 16(4):199–204. https://doi.org/10.1016/S0169-5347(01)02101-2
Kubát, K. (1990). Rumex L.-Štovík. Kvetena Ceské Republiky, 2:311–332.
Kwon O, Park J, Lee I-Y, Park J-E (2006) General biology of Ostrinia palustralis memnialis (Walker), a potential biological control agent of Rumex spp Korea. Entomol Res 36(3):179–182. https://doi.org/10.1111/j.1748-5967.2006.00030.x
Kwon O (2008) Status of weed biological control utilizing insects in Korea. Entomol Res. https://doi.org/10.1111/j.1748-5967.2008.00179.x
Lake JC, Leishman MR (2004) Invasion success of exotic plants in natural ecosystems: the role of disturbance, plant attributes and freedom from herbivores. Biol Cons 117(2):215–226. https://doi.org/10.1016/S0006-3207(03)00294-5
Lambert AM, Casagrande RA (2006) No evidence of fungal endophytes in native and exotic Phragmites australis. Northeast Nat 13(4):561–568. https://doi.org/10.1656/1092-6194(2006)13[561:neofei]2.0.co;2
Leigh EGJ (1997) Ecology of tropical forests: the view from Barro Colorado. Oxford University Press, Oxford
Leschen RAB, Lawrence JF, Kuschel G, Thorpe S, Wang Q (2003) Coleoptera genera of New Zealand. New Zealand Entomol 26(1):15–28. https://doi.org/10.1080/00779962.2003.9722105
Lieurance D, Cipollini D (2012) Damage levels from arthropod herbivores on Lonicera maackii suggest enemy release in its introduced range. Biol Invasions 14:863–873. https://doi.org/10.1007/s10530-011-0123-7
Liu H, Stiling P (2006) Testing the enemy release hypothesis: a review and meta-analysis. Biol Invasions 8:1535–1545. https://doi.org/10.1007/s10530-005-5845-y
Machado BB, Orue JP, Arruda MS, Santos CV, Sarath DS, Goncalves WN, Silva GG, Pistori H, Roel AR, Rodrigues-Jr JF (2016) BioLeaf: a professional mobile application to measure foliar damage caused by insect herbivory. Comput Electron Agric 129:44–55
Maron JL (1998) Insect herbivory above- and belowground: individual and joint effects on plant fitness. Ecology 79(4):1281. https://doi.org/10.2307/176743
Maron JL, Vila M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95(May):361–373. https://doi.org/10.1034/j.1600-0706.2001.950301.x
Martinková Z, Honěk A (2004) Gastrophysa viridula (Coleoptera: Chrysomelidae) and biocontrol of Rumex—A review. Plant Soil Environ 50(M):1–9
Meijer K, Schilthuizen M, Beukeboom L, Smit C (2016) A review and meta-analysis of the enemy release hypothesis in plant-herbivorous insect systems. PeerJ 2016(12):1–15. https://doi.org/10.7717/peerj.2778
Memmott J, Fowler SV, Paynter Q, Sheppard AW, Syrett P (2000) The invertebrate fauna on broom, Cytisus scoparius, in two, native and two exotic habitats. Acta Oecologica 21(3):213–222. https://doi.org/10.1016/S1146-609X(00)00124-7
Moore JP, Taylor JE, Paul ND, Whittaker JB (2003) Reduced leaf expansion as a cost of systemic induced resistance to herbivory. Funct Ecol 17:75–81. https://doi.org/10.1046/j.1365-2435.2003.00708.x
Morley TB, Faulkner S, Faithfull IG (2008) Establishment and dispersal of dock moth Pyropteron doryliformis ( Ochsenheimer) (Lepidoptera : Sesiidae ) in Victoria. In Proceedings of the 16th Australian weeds conference. Queensland Weeds Society, pp 272–274
Mukherjee A, Jones JW, Cuda JP, Kiker G, Overholt WA (2012) Effect of simulated herbivory on growth of the invasive weed Hygrophila polysperma: experimental and predictive approaches. Biol Control 60(3):271–279. https://doi.org/10.1016/j.biocontrol.2011.11.014
Myers JG, China WE (1928) XLIX—A list of New Zealand Heteroptera with the description of a remarkable green Aradid representing a new genus. Ann Mag Nat Hist 1(3):377–394. https://doi.org/10.1080/00222932808672798
Oliver I, Beattie AJ (1996) Invertebrate morphospecies as surrogates for species: a case study. Conserv Biol 10(1):99–109. https://doi.org/10.1046/j.1523-1739.1996.10010099.x
Orians CM, Ward D (2010) Evolution of plant defenses in nonindigenous environments. Annu Rev Entomol 55(1):439–459. https://doi.org/10.1146/annurev-ento-112408-085333
Park J, Lee IY, Park JE, Kwon O (2008) Allantus luctifer (Hymenoptera: Tenthredinidae), a candidate agent for the biological control of Rumex spp. Entomol Res 38(3):221–225. https://doi.org/10.1111/j.1748-5967.2008.00161.x
Pino J, Sans FX, Masalles RM (2002) Size-dependent reproductive pattern and short-term reproductive cost in Rumex obtusifolius L. Acta Oecologica 23(5):321–328. https://doi.org/10.1016/S1146-609X(02)01161-X
R Core Team (2019) R: a language and environment for statistical computing. Accessed from https://www.r-project.org/
Richardson DM, Pyšek P, Rejmánek M, Barbour MG, Dane Panetta F, West CJ (2000) Naturalization and invasion of alien plants: concepts and definitions. Divers Distrib 6:93–107. https://doi.org/10.1046/j.1472-4642.2000.00083.x
Schielzeth H (2010) Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1:103–113. https://doi.org/10.1111/j.2041-210X.2010.00012.x
Schultheis EH, Berardi AE, Lau JA (2015) No release for the wicked: enemy release is dynamic and not associated with invasiveness. Ecology 96(9):2446–2457. https://doi.org/10.1890/14-2158.1
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species. Biol Invasions 1(1):21–32. https://doi.org/10.1023/a:1010086329619
Spafford H, Hawley J, Strickland G (2008) Survival of dock moth larvae , Pyropteron doryliformis (Lepidoptera : Sesiidae ), in tubers of fiddle dock ( Rumex pulcher ). Weed Research
Torchin ME, Lafferty KD, Kuris AM (2001) Release from parasites as natural enemies: increased performance of a globally introduced marine crab. Biol Invasions 3(4):333–345. https://doi.org/10.1023/A:1015855019360
Turnipseed SG (1974) Sampling soybean insects by various d-vac, sweep, and ground cloth methods author. Fla Entomol 57(3):217–223
Uesugi A, Kessler A (2016) Herbivore release drives parallel patterns of evolutionary divergence in invasive plant phenotypes. J Ecol 104(3):876–886. https://doi.org/10.1111/1365-2745.12542
UK Natural History Museum. (2020). Accessed from https://www.nhm.ac.uk/content/dam/nhmwww/take-part/identification-trainers/coleoptera-families-guide.pdf
van Dam NM (2009) Belowground herbivory and plant defenses. Annu Rev Ecol Evol Syst 40(1):373–391. https://doi.org/10.1146/annurev.ecolsys.110308.120314
Van Der Putten WH, Bardgett RD, de Ruiter PC, Hol WHG, Meyer KM, Bezemer TM, Bradford MA, Christensen S, Eppinga MB, Fukami T, Hemerik L, Wardle DA (2009) Empirical and theoretical challenges in aboveground–belowground ecology. Oecologia 161(1):1–14
Van Lenteren JC, Babendreier D, Bigler F, Burgio G, Hokkanen HMT, Kuske S, Loomans AJM, Menzler-Hokkanen I, Van Rijn PCJ, Thomas MB, Tommasini MG, Zeng QQ (2003) Environmental risk assessment of exotic natural enemies used in inundative biological control. BioControl 48(1):3–38
Veselkin DV, Kuyantseva NB, Chashchina OE, Mumber AG, Zamshina GA, Molchanova DA (2019) Levels of leaf damage by phyllophages in invasive Acer negundo and native Betula pendula and Salix caprea. Russ J Ecol 50(6):511–516. https://doi.org/10.1134/S1067413619060134
Vilà M, Maron JL, Marco L (2005) Evidence for the enemy release hypothesis in Hypericum perforatum. Oecologia 142(3):474–479. https://doi.org/10.1007/s00442-004-1731-z
Williams JL, Auge H, Maron JL (2010) Testing hypotheses for exotic plant success: parallel experiments in the native and introduced ranges. Ecology 91(5):1355–1366. https://doi.org/10.1890/08-2142.1
Williamson MH, Fitter A (1996) The characters of successful invaders. Biol Cons 78(1–2):163–170. https://doi.org/10.1016/0006-3207(96)00025-0
Willis AJ, Memmott J, Forrester RI (2000) Is there evidence for the post-invasionevolution of increased size among invasive plant species? Ecol Lett 3:275–283
Zangerl AR, Hamilton JG, Miller TJ, Crofts AR, Oxborough K, Berenbaum MR, De Lucia EH (2002) Impact of folivory on photosynthesis is greater than the sum of its holes. Proc Natl Acad Sci USA 99(2):1088–1091. https://doi.org/10.1073/pnas.022647099
Zangerl AR, Stanley MC, Berenbaum MR (2008) Selection for chemical trait remixing in an invasive weed after reassociation with a coevolved specialist. Proc Natl Acad Sci USA 105(12):4547–4552. https://doi.org/10.1073/pnas.0710280105
Zvereva EL, Kozlov MV (2012) Sources of variation in plant responses to belowground insect herbivory: a meta-analysis. Oecologia 169(2):441–452. https://doi.org/10.1007/s00442-011-2210-y
Acknowledgements
We would like to thank the following people for their help in conducting the field surveys: Hamish Robb, Sylvester Atijegbe and Francesco Martoni. We also thank the following people and organizations who helped us locate populations and gave permission for collection: Botanical Society of the British Isles, Scottish Natural Heritage and Historic Environment Scotland, Christchurch and Invercargill City Councils, Taane Johnsen & Colin Ferguson, and numerous farm owners and managers around New Zealand.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This project was supported by the New Zealand Tertiary Education Commission CoRE grant to the Bio-Protection Research Centre.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Philip E. Hulme obtained the research funding. Data collection and analysis were performed by Cristian-Andrei Costan. The first draft of the manuscript was written by Cristian-Andrei Costan and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Costan, CA., Godsoe, W.K., Bufford, J.L. et al. Can the enemy release hypothesis explain the success of Rumex (Polygonaceae) species in an introduced range?. Biol Invasions 24, 2665–2677 (2022). https://doi.org/10.1007/s10530-022-02810-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-022-02810-w