Summary
The projections to the midbrain from the spinal cord have been investigated in the cat with the degeneration technique and by using horseradish peroxidase (HRP) as an anterograde tracer. Two types of spinal cord lesions were performed: 1) Cordotomies at cervical or thoracic levels transecting the ventral and lateral funiculi. 2) Transections of the ventral, ventrolateral, dorsolateral or dorsal funiculus, respectively, at cervical levels. In the anterograde tracing experiments HRP was injected into the spinal cord at cervical, lumbar or sacral levels.
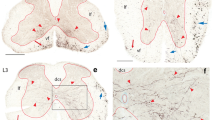
The results show large projections to the lateral and ventrolateral parts of the periaqueductal gray (PAG1), the posterior pretectal nucleus (PP) and the nucleus of Darkschewitsch (D). More moderate projections go to the medial division of the periaqueductal gray (PAGm), the cuneiform nucleus (CF), the mesencephalic reticular formation (MRF), lateral part of the deep layer of the superio colliculus (SP) and magnocellular medial geniculate nucleus (GMmc), while scattered spinal fibers are present in the dorsal part of the periaqueductal gray (PAGd), the external inferior collicular nucleus (IX), the intermediate layer of the superior colliculus (SI), the lateral part of the red nucleus (NR) and in the Edinger-Westphal portion of the oculomotor nucleus (3). In addition a few fibers are present in the interstitial nucleus of Cajal (CA) and anterior pretectal nucleus (PAc).
The results indicate that at midcervical levels most of the spinomesencephalic fibers ascend in the ventral funiculus, with only a moderate fraction ascending in the ventral half of the lateral funiculus. Almost no fibers ascend in the dorso-lateral funiculus and none appear to pass in the dorsal funiculus.
No distinct somatotopic pattern was found in the spinomesencephalic projections, but more fibers from cervicobrachial segments terminate in the rostral than in the caudal parts of the terminal fields in PAG, CF, SP and IX, while the lumbar fibers were more numberous in the caudal parts. PP seems to receive spinal fibers mainly from the caudal half of the cord.
Similar content being viewed by others
References
Aitkin LM, Dickhaus H, Schult W, Zimmerman M (1978) External nucleus of the inferior colliculus: Auditory and spinal somatosensory afferents and their interactions. J Neurophysiol 41:837–847
Aitkin LM, Kenyon CE, Philpott P (1981) The representation of the auditory and somatosensory systems in the external nucleus of the inferior colliculus. J Comp Neurol 196:25–40
Andersson FD, Berry CM (1959) Degeneration studies of long ascending fiber systems in the cat brain stem. J Comp Neurol 111:195–229
Antonetty CM, Webster KE (1975) The organization of the spinotectal projection. An experimental study in the rat. J Comp Neurol 163:449–466
Applebaum AE, Beall JE, Foreman RD, Willis WD (1975) Organization and receptive fields of primate spinothalamic tract neurons. J Neurophysiol 38:572–586
Avendano C, Juretschke MA (1980) The pretectal region of the cat: A structural and topographical study with stereotaxic coordinates. J Comp Neurol 193:69–88
Basbaum AI (1980) The anatomy of pain and pain modulation. In: Kosterlitz HW, Terenius LY (eds). Pain and society. Verlag Chemie, Weinheim, pp 93–122
Basbaum AI, Fields HL (1979) The origin of descending pathways in the dorsolateral funiculus of the spinal cord of the cat and rat: Further studies on the anatomy of pain modulation. J Comp Neurol 187:513–532
Beitz AJ (1982) The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience 7:133–159
Berman AL (1968) Thr brain stem of the cat. A cytoarchitectonic atlas with stereotaxic coordinates. Univ of Wisconsin Press, Madison
Besson JM, Guilbaud G, Abdelmoumene M, Chaouch A (1982) Physiologie de la nociception. J Physiol (Paris) 78:7–107
Björkeland M, Boivie J (1984a) Anatomy of the midbrain projections from the lateral cervical nucleus in cat. In: Rowe M, Willis WD (eds). Development, organization and processing in somatosensory pathways. A. Liss, New York (in Press)
Björkeland M, Boivie J (1984b) An anatomical study of the projections from the dorsal column nuclei to the midbrain in cat. Anat Embryol 170:29–43
Bowsher D (1957) Termination of the central pain pathway in man: The conscious appreciation of pain. Brain 80:606–622
Bowsher D (1976) Role of the reticular formation in responses to noxious stimulation. Pain 2:361–378
Cervero F, Iggo A, Molony V (1977) Responses of spinocervical tract neurones to noxious stimulation of the skin. J Physiol 267:537–558
Christensen BN, Perl ER (1970) Spinal neurons specifically excited by noxious or thermal stimuli: Marginal zone of the dorsal horn. J Neurophysiol 33:293–307
Collier J, Buzzard EF (1903) The degenerations resulting from lesions of posterior nerve roots and from transverse lesions of the spinal cord in man. A study of twenty cases. Brain 26:559–591
Fink RP, Heimer L (1967) Two methods for selective silver impregnation of degenerating axons and their synaptic endings in the central nervous system. Brain Res 4:369–374
Flink R, Wiberg M, Blomqvist A (1983) The termination in the mesencephalon of fibers from the lateral cervical nucleus. An anatomical study in the cat. Brain Res 259:11–20
Gacek RR (1971) Anatomical demonstration of the vestibulo-ocular projections in the cat. Acta Orolaryngol (Stockh) Suppl 293:1–63
Giesler GJ, Liebeskind JC (1976) Inhibition of visceral pain by electrical stimulation of the periaqueductal gray matter. Pain 2:43–48
Gordon B (1973) Receptive fields in deep layer of cat superior colliculus. J Neurophysiol 36:157–178
Gros Clark Le WE (1936) The termination of ascending tracts in the thalamus of the macaque monkey. J Anat (London) 71:7–40
Hamilton BL (1973) Cytoarchitectural subdivisions of the periaqueductal gray matter in the cat. J Comp Neurol 149:1–28
Hardy SGP, Haigler HJ, Leichnetz GR (1983) Paralemniscal reticular formation: Responses of cells to a noxious stimulus. Brain Res 267:217–223
Harris LR (1980) The superior colliculus and movements of the head and eyes in cats. J Physiol 300:367–391
Hunsperger RW (1956) Role of substantia grisea centralis mesencephali in electrically induced rage reactions. In: Kappers Ariens J (ed) Progress in neurobiology. Vol I. Elsevier New York, pp 289–294
Johnson FH (1954) Experimental study of spino-reticular connections in the cat. Anat Rec 118:316
Jurgens U, Pratt R (1979) Role of the periaqueductal grey in vocal expression of emotion. Brain Res 167:367–378
Kanaseki T, Sprague JM (1974) Anatomical organization of pretectal nuclei and tectal laminae in the cat. J Comp Neurol 158:319–338
Kawamura S, Niimi K (1972) Counterstaining of Nauta-Gygax impreganted sections with cresyl violet. Stain Technol 47:1–16
Kerr FWL (1975) The ventral spinothalamic tract and other ascending systems of the ventral funiculus of the spinal cord. J Comp Neuro 159:335–356
Kerr FWL, Lippman HH (1974) Thr primate spinothalamic tract as demonstrated by anterolateral cordotomy and commissural myelotomy. Adv Neurol 4:147–156
Killackey HP, Erzurumku RS (1981) Trigeminal projections to the superior colliculus of the rat. J Comp Neurol 201:221–242
King WM, Fuchs AF, Magnin M (1981) Vertical eye movementrelated responses of neurons in midbrain near interstitial nucleus of Cajal. J Neurophysiol 46:549–562
Liu RPC (1983) Laminar origin of spinal projection neurons to the periaqueductal gray of the rat. Brain Res 264:118–122
Loewy AD, Saper CB (1978) Edinger-Westphal nucleus: Projections to the brain stem and spinal cord in the cat. Brain Res 150:1–27
Matyh PW (1982a) The ascending input to the midbrain periaqueductal grey of the primate. J Comp Neurol 211:50–64
Mantyh PW (1982b) The midbrain periaqueductal gray in the rat, cat and monkey: A Nissl, Weil and Golgi analysis. J Comp Neuro 204:349–363
Mantyh PW, Peschanski M (1982) Spinal projections from the periaqueductal grey and dorsal raphe in the rat, cat and monkey. Neuroscience 7:2769–2776
Mayer DJ, Price DD, Becher DP (1975) Neurophysiological characterization of the anterolateral spinal cord neurons contributing to pain perception in man. Pain 1:51–58
Mehler WR (1957) The mammalian “Pain tract” in phylogeny. Anat Rec 127:332
Mehler WR (1962) The anatomy of the so called “pain tract” in man. An analysis of the course and distribution of the ascending fibers the fasciculus anterolateralis. In: French JD, Porter RW (eds). Basic research in paraplegia. Thomas, Springfield, pp 26–55
Mehler WR (1966) Some observations on secondary ascending afferent systems in the central nervous system. In: Knighton RS, Dumke PR (eds). Pain. Little Brown, Boston
Mehler WR (1969) Some neurological species differences. A posteriori. Ann NY Acad Sci 169:424–468
Mehler WR, Feferman ME, Nauta WJH (1960) Ascending axon degeneration following antero-lateral cordotomy. An experimental study in the monkey. Brain 83:718–750
Menétrey D, Chaouch A, Binder D, Besson JM (1982) The origin of the spinomesencephalic tract in the rat: An anatomical study using the retrograde transport of horseradish peroxidase. J Comp Neurol 206:193–207
Mesulam MM (1978) Tetramethylbenzidine for horseradish peroxidase neurochemistry: A non-carcinogenic blue product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Moss MS, Basbaum AI (1983) The peptidergic organization of the cat periaqueductal gray. II. The distribution of immunoreactive substance P and vasoactive intestinal polypeptide. J Neurosci 3:1437–1449
Moss MS, Glazer EJ, Basbaum AI (1983) The peptidergic organization of the cat periaqueductal gray. I. The distribution of immunoreactive enkephalin-containing neurons and terminals. J Neurosci 3:603–616
Mott FW (1892) Ascending degenerations resulting from lesions of the spinal cord in monkeys. Brain 15:215–229
Mott FW (1895) Experimental enquiry upon the afferent tracts of the central nervous system of the monkey. Brain 18:1–20
Nagata T, Kruger L (1979) Tactile neurons of the superior colliculus of the cat: Input and physiological properties. Brain Res 174:19–37
Nakahama H, Shima K, Aya K, Fuji H (1981) Peripheral somatic activation and spontaneous firing patterns of neurons in the periaqueductal gray of the cat. Neurosci Lett 25:43–46
Nakamuta Y, Kitao Y, Okoyama S (1983) Projections from the pericruciate cortex to the nucleus of Darkschewitsch and other structures at the mesodiencephalic junction in the cat. Brain Res Bull 10:517–521
Nashold BS, Wilson WP, Slaughter DG (1969) Sensations evoked by stimulation in the midbrain of man. J Neurosurg 30:14–24
Nauta WJH, Kuypers HGJM (1958) Some ascending pathways in the brain stem reticular formation. In: Jasper HH, Proctor LD (eds). Reticular formation of the brain. Little Brown, Boston pp 3–30
Nijensohn DE, Kerr FWL (1975) The ascending projections of the dorsolateral funiculus of the spinal cord in the primate. J Comp Neurol 161:459–470
Phipps BS, Maciewicz R, Sandrew BB, Poletti CE, Foote WE (1983) Edinger-Westphal neurons that project to spinal cord contain substance P. Neurosci Lett 36:125–131
Pompeiano O, Walberg F (1957) Descending connections to the vetibular nuclei. An experimental study in the cat. J Comp Neurol 108:465–503
RoBards MJ, Watkins DW, Masterton RB (1976) An anatomical study of some somesthetic afferents to the intercollicular terminal zone of the midbrain of the opossum. J Comp Neurol 170:499–524
Roucoux A, Guitton D, Crommelinck M (1980) Stimulation of the superior colliculus in the alert cat. II. Eye and head movements evoked when the head is unrestrained Exp Brain Res 39:75–85
Saint-Cyr JA, Courville J (1980) Projections from the motor cortex, midbrain and vestibular nuclei to the inferior olive in the cat: Anatomical organization and functional correlates. In: Courville J, Montigny C, Lamarre Y (eds). The inferior olivary nucleus. Raven Press. New York pp 97–124
Scheibel A, Markham CH, Koegler R (1961) Neural correlates of the vestibulo-ocular reflex. Neurology 11:1055–1065
Schroeder DM, Jane JA (1976) The intercollicular area of the inferior colliculus. Brain Behav Evol 13:125–141
Sprague JM, Berlucchi G, Rizzolatti G (1973) The role of the superior colliculus and pretectum in vision and visually guided behaviour. In: Jung R (ed). Visual centres in the brain. Handbook of sensory physiology. Vol VII/3. Central processing of visual information, part B. Springer, Berlin pp 27–101
Stein BE, Dixon JP (1978) Superior colliculus cells respond to noxious stimuli. Brain Res 158:65–73
Stein BE, Magalhaes-Castro B, Kruger L (1976) Relationship between visual and tactile representations in cat superior colliculus. J Neurophysiol 39:401–419
Sugimoto T, Mizuno N, Uchida K (1982) Distribution of cerebellar fiber terminals in the midbrain visuomotor areas: An autoradiographic study in the cat. Brain Res 238:353–370
Taber E (1961) The cytoarchitecture of the brain stem of the cat. I. Brain stem nuclei of cat. J Comp Neurol 116:27–69
Tashiro T, Kudo M, Kawamura S (1980) Discontinuous spatial distribution of the tectal afferents from the trigeminal nucleus in the cat. Neurosci Lett 20:249–252
Tawil RN, Saadé NE, Bitar M, Jabbur SJ (1983) Polysensory interactions on single neurons of cat inferior colliculus. Brain Res 269:149–152
Walberg F (1982) The origin of olivary afferents from the central grey ant its surroundings in the cat. Anat Embryol 164:139–151
Walker AE (1938) The thalamus of the chimpanzee. I. Terminations of the somatic afferent systems. Confinia Neurol 1:99–127
Walker AE (1940) The spinothalamic tract in man. Arch Neurol Psychiat 43:284–298
Wiberg M, Blomqvist A (1984) The spinomesencephalic tract in the cat: Its cells of origin and termination pattern as demonstrated by the intraaxonal transport method. Brain Res 291:1–18
Wiitanen JT (1969) Selective silver impregnation of degenerating axons and axon terminals in the central nervous system of the monkey (Macaca mulatta). Brain Res 14:546–548
Willis WD, Maunz RA, Foreman RD, Coulter JD (1975) Static and dynamic responses of spinothalamic tract neurons to mechanical stimuli. J Neurophysiol 38:587–600
Zemlan FP, Leonard CM, Kow L-M, Pfaff DW (1978) Ascending tracts of the lateral columns of the rat spinal cord: A study using the silver impregnation and horseradish peroxidase techniques. Exp Neurol 62:298–334
Zuk A, Rutherford JG, Gwyn DG (1983) Projections from the interstitial nucleus of Cajal to the inferior olive and to the spinal cord in cat: A retrograde fluorescent double-labeling study. Neurosci Lett 38:95–101
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Björkeland, M., Boivie, J. The termination of spinomesencephalic fibers in cat. Anat Embryol 170, 265–277 (1984). https://doi.org/10.1007/BF00318730
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00318730