Abstract
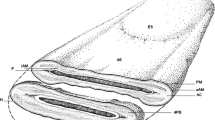
A polyclonal rabbit antibody against 5′-nucleotidase purified from bull seminal plasma was used to localize the antigen on bovine spermatozoa. Spermatozoa taken from the ampulla of the vas deferens showed strong immunofluorescence at the anterior rim of the head portion. Evaluation of spermatozoa prepared from different segments of the seminal pathway indicated the presence of the antigen already in rete testis and epididymal spermatozoa. On cryostat sections of testis tissue a positive immunoreaction was found in the anterior head portion of elongated spermatids, but not in earlier forms of sperm development. This distribution corresponded with the enzyme activity and results of Western blotting in extracts of testicular and epididymal spermatozoa. Immunoelectron microscopy of ampullary spermatozoa using antibody detection with gold-labelled anti-rabbit IgG showed a clear-cut labelling of the plasma membrane in the acrosome region. Treatment of ampullary spermatozoa with 0.1% Triton X-100 did not completely remove the immunoreactive material from the acrosome, showing a very stable linkage of the protein to the plasma membrane. Treatment with phospholipase C from Bacillus thuringiensis, however, removed immunoreactive material from the plasma membrane, indicating its binding by a phosphoinositol anchor. Our findings show that endogenous 5′-nucleotidase is present on the plasma membrane covering the anterior head portion of bovine spermatozoa and indicate specialized functions during the acrosomal reaction. Soluble enzyme derived from seminal vesicle secretion covers the whole sperm surface during emission, but is not covalently bound. It provides generalized enzyme activity to the sperm surface in addition to the specialized area of the sperm head.
Similar content being viewed by others
References
Agrawal Y, Vanha-Perttula T (1987) Effect of secretory particles in bovine seminal vesicle secretion on sperm motility and acrosome reaction. J Reprod Fertil 79:409–419
Allison RC, Hernandez EM, Prasad VR, Grisham MB, Taylor AE (1988) Protective effects of O2 radical scavengers and adenosine in PMA-induced lung injury. J Appl Physiol 64:2175–2182
Aumüller G, Riva A (1992) Morphology and functions of the human seminal vesicle. Andrologia 24:183–196
Aumüller G, Seitz J (1990) Protein secretion and secretory processes in male accessory sex gland. Int Rev Cytol 121:127–231
Aumüller G, Scheit KH (1987) Immunohistochemistry of secretory proteins in the bull seminal vesicle. J Anat 150:43–48
Aumüller G, Vesper M, Seitz J, Kemme M, Scheit KH (1988) Binding of a major secretory protein from bull seminal vesicles to bovine spermatozoa. Cell Tissue Res 252:377–384
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248
Čéchová D, Töpfer-Petersen E, Henschen A (1988) Boar acrosin is a single-chain molecule which has the N-terminus of the acrosin A-chain (light chain). FEBS Lett 241:136–140
Coronel CE, Lardy HA (1992) Functional properties of caltrin proteins from seminal vesicle of the guinea pig. Mol Reprod Dev 33:74–80
Corthier G, Boschetti E, Charley-Poulain J (1984) Improved method for IgG purification from various animal species by ion exchange chromatography. J Immunol Methods 66:75–79
d'Alessio G, Floridi A, de Prisco R, Pignero A, Leone E (1972) Bull semen ribonucleases. 1. Purification and physico-chemical properties of the major component. Eur J Biochem 26:153
Dieckhoff J, Heidemann M, Lietzke R, Mannherz HG (1986) Isolation and characterization of 5′-nucleotidase from avian muscle sources and its interaction with filamentous actin. In: Kreutzberg GW, Reddington M, Zimmermann H (eds) Cellular biology of ectoenzymes. Springer, Berlin Heidelberg New York, pp 133–146
Fini C, Cannistrado S (1990) 5′-Nucleotidase from bull seminal plasma. Biochemical and biophysical aspects. Andrologia 22:33–43
Fini C, Ipata PL, Palmerini CA, Floridi A (1983) 5′-Nucleotidase from bull seminal plasma. Biochim Biophys Acta 748:405–412
Gadella BM, Colenbrander B, Golde LMG van, Lopes-Cardozo M (1993) Boar seminal vesicles secrete arylsulfatases into seminal plasma: evidence that desulfation of seminolipid occurs only after ejaculation. Biol Reprod 48:483–489
Gower HJ, Barton CH, Elsom VL, Thompson J, Moore SE, Dickson G, Walsh FS (1988) Alternative splicing generates a secreted form of N-CAM in muscle and brain. Cell 55:955–964
Graham RC, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubule of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem 14:291–302
Heppel LA, Hilmoe RJ (1951) Purification and properties of 5′-nucleotidase. J Biol Chem 188:665–676
Hinsch K-D, Hinsch E, Aumüller G, Tychowiecka I, Schultz G, Schill W-B (1992) Immunological identification of G protein α- and β-subunits in tail membranes of bovine spermatozoa. Biol Reprod 47:337–346
Ipata PL (1967) A coupled optical enzyme assay for 5′-nucleotidase. Anal Biochem 20:30–36
Lemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
LeHir M, Kaissling B (1989) Distribution of 5′-nucleotidase in the renal interstitium of the rat. Cell Tissue Res 258:177–182
LeHir M, Kaissling B (1993) Distribution and regulation of renal ecto-5′-nucleotidase: implications for physiological functions of adenosine. Am J Physiol 264 (Renal Fluid Electrolyte Physiol 33): F377-F387
Levin SJ, Bodansky O (1966) The double pH optimum of 5′-nucleotidase of bull seminal plasma (1966) J Biol Chem 241:51–56
Lilja H, Oldbring J, Rannevik G, Laurell C-B (1987) Seminal-vesicle secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest 80:281–285
Mann T (1954) The biochemistry of semen, 1st edn. Methuen, London
Meistrich ML, Bruce WR, Clermont Y (1973) Cellular composition of fractions of mouse testis cells following velocity sedimentation separation. Exp Cell Res 79:213–227
Moore A, Penfold LM, Johnson JL, Latchman DS, Moore HDM (1993) Human sperm-egg binding is inhibited by peptides corresponding to core region of an acrosomal serine protease inhibitor. Mol Reprod Dev 34:280–291
Rönkkö S (1992) Immunohistochemical localization of phospholipase A2 in the bovine seminal vesicle and on the surface of the ejaculated spermatozoa. Int J Biochem 24:869–876
Rufo GA Jr, Singh JP, Babcock DF, Lardy HA (1982) Purification and characterization of a calcium transport inhibitor protein from bovine seminal plasma. J Biol Chem 257:5627–4632
Sanz L, Calvete JJ, Mann K, Schäfer W, Schmid ER, Töpfer-Petersen E (1992) The complete primary structure of the boar spermadhesin AQN-1, a carbohydrate-binding protein involved in fertilization. Eur J Biochem 205:645–652
Sanz L, Calvete JJ, Mann K, Gabius H-J, Töpfere-Petersen E (1993) Isolation and biochemical characterization of heparinbinding proteins from boar seminal plasma: a dual role for spermadhesins in fertilization. Mol Reprod Dev 35:37–43
Shivaji S (1984) Antimicrobial activity of semen. Trends Biochem Sci 9:104–107
Shivaji S, Scheidt KH, Bhargawa P (1990) Proteins of the seminal fluid. Wiley, London
Stochaj U, Dieckhoff J, Mollenhauer J, Cramer M, Mannherz HG (1989) Evidence for the direct interaction of chicken gizzard 5′-nulceotidase with laminin and fibronectin. Biochim Biophys Acta 992:385–392
Takata K, Kasahara T, Kasahara M, Ezaki O, Hirano H (1992) Immunohistochemical localization of Na+-dependent glucose transporter in rat jejunum. Cell Tissue Res 267:3–9
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Ward PA, Cunningham TW, McCulloch KK, Johnson KJ (1988) Regulatory effects of adenosine and adenine nucleotides on oxygen radical responses of neutrophils. Lab Invest 58:438–447
Zimmermann H (1992) 5′-Nucleotidase: molecular structure and functional aspects. Biochem J 285:345–365
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schiemann, P.J., Wennemuth, G., Aumüller, G. et al. Distribution of endogenous and exogenous 5′-nucleotidase on bovine spermatozoa. Histochemistry 101, 253–262 (1994). https://doi.org/10.1007/BF00315912
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315912