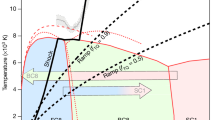
Abstract
Diamond (∼1 μm) and graphite (1–10 μm) in NaCl were melted at 50 to 300 kbar in a diamond anvil cell using a pulsed YAG laser. The samples were removed from the cell and the structures of the quenched phases were studied by transmission electron microscopy.
The melted regions of the samples were found to consist of nearly perfect spheres of carbon ranging in size from ∼1 μm down to less than a few nanometers. In the diamond sample melted at 300 kbar, the larger spherules (>0.2 μm) are polycrystalline diamond with either a granular or radial texture. The smaller spherules (<0.2 μm) give electron diffraction patterns with four diffuse rings that correspond to the 002, 100, 004 and 110 of graphite. This diffraction pattern is typical of disordered graphite randomly oriented about the c-axis. Dark field imaging, using a portion of the 002 ring, produces a “bow tie” figure in each of the smaller spherules. The orientation of the “bow tie” figure depends on the portion of the ring used to form the image, and indicates a radial orientation of the c-axis of the disordered graphite. The spacing between the 002 layers depends on the pressure at the time of melting. We interpret this to indicate that there is some sp3 bonding between layers in the disordered graphite in the smaller spherules. The smaller spherules may have the disordered graphite structure because of the effect of the size on the free energy relationship between the phases, or they may have been quenched more rapidly than the larger spherules thus preserving some of the character of the melt. If the latter explanation is correct, then our results may indicate that the diamond melt contains significant sp2 bonding.
Lattice images (Fig. 12) of the internal structure of the smallest spherules observable (∼50 A) clearly show that the carbon layers are parallel to the surface of the spherules and that there is a great deal of disorder in the layers. These observations are entirely consistent with our conclusions based on the dark field images.
Similar content being viewed by others
References
Bundy FP (1962) Melting point of graphite at high pressure: heat of fusion. Science 137:1055–1058
Bundy FP (1963) Melting of graphite at very high pressure. J Chem Phys 38:618–630
Bundy FP (1980) The P,T phase and reaction diagram for elemental carbon, 1979. J Geophys Res 85:6930–6936
Bundy FP, Kasper JS (1967) Hexagonal diamond — A new form of carbon. J Chem Phys 46:3437–3446
Dickey JS Jr, Bassett WA, Bird JM, Weathers MS (1983) Liquid carbon in the lower mantle? Geology 11:219–220
Elman BS, Dresselhaus MS, Braunstein G, Dresselhaus G, Venkatesan T, Wilkens B, Gibson JM (1984) Two-dimensional ordering of ion damaged graphite. Mat Res Soc Symp Proc 27:461–466
Gold JS, Bassett WA, Weathers MS, Bird JM (1984) Melting of diamond. Science 225:921–922
Grover R (1979) Does diamond melt? J Chem Phys 71:3824–3829
Kleiman J, Heimann RB, Hawken D, Salansky NM (1984) Shock compression and flash heating of graphite/metal mixtures at temperatures up to 3200 K and pressures up to 25 GPa. J Appl Phys 56:1440–1454
Kleiman J, Salansky NM, Heiman RB (1984) Nucleation, growth and regraphitization of diamonds and other carbonaceous materials formed in flash-heating implosion experiments. Mat Res Soc Symp Proc 22:223–226
Ree FH, van Thiel M, Calef D (1987) Stability of graphite and diamond clusters and their transformation rates under high pressure and temperature. Bull Am Phy Soc 32:607
Shaner JW, Brown JM, Swenson CA, McQuenn RG (1984) Sound velocity of carbon at high pressures. J Phys 45:C8 235–237
Van Vechten JA (1973) Quantum dielectric theory of electronegativity in covalent systems. III. Pressure-temperature phase diagrams, heats of mixing, and distribution coefficients. Phys Rev B7:1479–1507
Yin MT, Cohen ML (1983) Will diamond transform under megabar pressures? Phys Rev Lett 50:2006–2009
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weathers, M.S., Bassett, W.A. Melting of carbon at 50 to 300 kbar. Phys Chem Minerals 15, 105–112 (1987). https://doi.org/10.1007/BF00308772
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00308772