Summary
-
1.
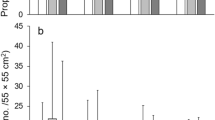
The oxygen consumption of the sand crab, Emerita asiatica, when estimated employing solitary specimens showed an unmistakable persistent tidal rhythm.
-
2.
Numbers of 4 and 5 crabs even when huddled together in the respiration chambers showed the rhythmicity in their metabolic rates indicating mutual synchronisation of individual oscillations.
-
3.
In newly moult crabs, in spite of the intensified level of metabolism accompanying the process of moulting, the tidal rhythms were displayed in the metabolic rates.
-
4.
Simultaneous estimations of the swimming activity employing a vertically moving cage device and the oxygen consumption of individual crabs further confirmed the persistence of rhythms.
-
5.
The activity of Emerita coinciding with the high tide at night was considerably enhanced. It was clear that this exaggerated nightly activity was due to the superimposition of a diurnal rhythm an a tidal rhythm.
-
6.
The rhythms in the locomotory activity waned after the crabs had been in the laboratory for 3–4 days.
-
7.
The behaviour of Emerita, as seen in the activity records and oxygen consumption estimations made in the present study, is reminiscent of its behaviour in nature relative to the tide.
-
8.
The adaptive significance of such rhythmic behaviour to the continued existence of littoral animals is evident.
Zusammenfassung
-
1.
Emerita asiatica hat einen deutlichen Gezeitenrhythmus des Sauerstoffverbrauchs, der auch unter Laboratoriumsbedingungen weiterläuft.
-
2.
Werden mehrere Individuen in Gemeinschaft gehalten, so zeigt sich eine gegenseitige Synchronisation hinsichtlich dieser Stoffwechselschwankungen.
-
3.
Frisch gehäutete Individuen zeigen, trotz des verstärkten Stoffwechsels während der Häutung, diese Gezeitenrhythmik ebenfalls.
-
4.
Gleichzeitige Messung der Schwimmaktivität und des Stoffwechsels bestätigte das Fortdauern der Rhythmik unter Laboratoriums bedingungen.
-
5.
Zu den Zeiten nächtlicher Flut ist die Bewegungsaktivität erheblich verstärkt. Das beruht deutlich auf einer Überlagerung der diurnalen Rhythmik mit der Gezeitenrhythmik.
-
6.
Nach 3–4 Tagen des Laboratoriumsaufenthaltes zeigt sich bei der Bewegungsaktivität eine Dämpfung der Rhythmik.
-
7.
Das Verhalten im Laboratorium ist sowohl hinsichtlich des Sauerstoffverbrauches als hinsichtlich der Schwimmaktivität eine Fortsetzung des dem natürlichen Gezeitenwechsel angemesssenen Verhaltens.
-
8.
Die ökologische Bedeutung dieser rhythmischen Verhaltensweisen unter den Lebensbedingungen an der Küste ist evident.
Similar content being viewed by others
Bibliography
Abramowitz, R.K., and A.A. Abramowitz: Moulting, growth and survival after eyestalk removal in Uca pugilator. Biol. Bull. 78, 179–188 (1940).
Aschoff, J.: Comparative Physiology: Diurnal rhythms. Ann. Rev. Physiol. 25, 581–600 (1963).
Bennett, M.F., J. Shriner, and R.A. Brown: Persistent tidal cycles of spontaneous motor activity in the fiddler crab, Uca pugnax. Biol. Bull. 112, 267–275 (1957).
Blume, J., E. Bünning u. D. Müller: Periodenanalyse von Aktivitätsrhythmen bei Carcinus maenas. Biol. Zbl. 81, 569–573 (1962).
Bohn, G.: Sur les mouvements oscillatoires de Convoluta roscoffensis. C. R. Acad. Sci. (Paris) 137, 576–578 (1903).
—: Periodicité vitale des animaux soumis aux oscillations du niveau des hautes mers. C. R. Acad. Sci. (Paris) 139, 610–611 (1904).
Brown F.A., jr., M. Fingerman, M.I. Sandeen, and H.M. Webb: Persistent diurnal and tidal rhythms of color change in the fiddler crab, Uca pugnax. J. exp. Zool. 123, 29–60 (1953).
Bünning, E.: Die physiologische Uhr, 2. Aufl. Berlin-Göttingen-Heidelberg: Springer 1963.
Cloudsley-Thompson, J.L.: Rhythmic activity in animal physiology and behaviour. New York and London: Academic Press 1961.
Cole, L.C.: Biological clock in the unicorn. Science 125, 874–876 (1957).
Enright, J.T.: The tidal rhythm of activity of a sand-beach amphipod. Z. vergl. Physiol. 46, 276–313 (1963).
Fingerman, M.: Persistent daily and tidal rhythms of color change in Callinectes sapidus. Biol. Bull. 109, 255–264 (1955).
—: Tidal rhythmicity in marine organisms. Cold Spr. Harb. Symp. quant. Biol. 25, 481–489 (1960).
Gamble, F.W., and F. Keeble: The bionomics of Convoluta roscoffensis with special reference to its green cells. Proc. Roy. Soc. B 72, 93–98 (1903).
—: The bionomics of Convoluta roscoffensis with special reference to its green cells. Quart. J. micr. Sci. 47, 363–431 (1904).
Gompel, M.: Recherches sur la consommation d'oxygene de quelques animaux aquatiques littoraux. C. R. Acad. Sci. (Paris) 205, 816–818 (1937).
Harker, J.E.: Diurnal rhythms in the animal kingdom. Biol. Rev. 33, 1–52 (1958).
Hauenschild, C.: Lunar periodicity. Cold Spr. Harb. Symp. quant. Biol. 25, 491–497 (1960).
Job, S.V.: The oxygen consumption of Salvelinus fontinalis. Ph. D. Thesis, Dep. of Zool., University Toronto 1957.
MacGinitie, G.E., and N. MacGinitie: Natural history of marine animals. New York: MacGraw-Hill Book Co. 1949.
Naylor, E.: Tidal and diurnal rhythms of locomotory activity in Carcinus maenas (L.). J. exp. Biol. 35, 602–610 (1958).
—: Locomotory rhythms in Carcinus maenas. J. exp. Biol. 37, 481–488 (1960).
Pearse, A.S., H.J. Humm, and G.W. Wharton: Ecology of sand beaches at Beaufort, N. C. Ecol. Monogr. 12, 135–190 (1942).
Roberts, J.L.: Thermal acclimation of metabolism in the crab, Pachygrapsus crassipes Randall. 1. The influence of body size, starvation and moulting. Physiol. Zool. 30, 232–242 (1957).
Sandeen, M.I., G.C. Stephens, and F. A. Brown jr: Persistent daily and tidal rhythms of oxygen consumption in two species of marine snails. Physiol. Zool. 27, 350–356 (1954).
Schlaifer, A.: Oxygen consumption of goldfish. Physiol. Zool. 12, 381–392 (1939).
Schuett, F.: Studies in mass physiology. The effects of numbers on the oxygen consumption of fishes. Ecology 14, 106–122 (1933).
Turner, H.J., and D.L. Belding: The tidal migration of Donax variabilis. Contribution No 886, Woodshole (Mass.) 1957.
Webb, H.M., and F.A. Brown jr.: Timing long cycle physiological rhythms. Physiol. Rev. 39, 127–161 (1959).
Wieser, W.: Adaptations of two intertidal isopods. 1. Respiration and feeding in Naesa bidentata (Adams). J. mar. biol. Ass., U.K. 42, 665–682 (1962).
Wolf, W. (Ed.): Rhythmic functions in the living system. Ann. N. Y. Acad. Sci. 98, 753–1326 (1962).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chandrashekaran, M.K. Persistent tidal and diurnal rhythms of locomotory activity and oxygen consumption in Emerita asiatica (M.-EDW.). Z. Vergl. Physiol. 50, 137–150 (1965). https://doi.org/10.1007/BF00302701
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00302701