Abstract
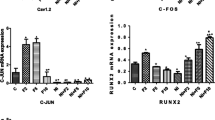
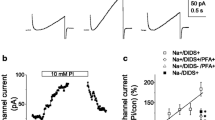
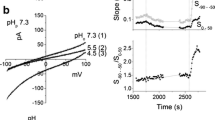
The present study evaluates differential occurrence of voltage-dependent calcium channels (VDCC) in the membranes of fetal (FROB) and neonatal (NROB) calvarian rat osteoblastic cells in primary culture. The intracellular calcium concentration ([Ca2+]i) was monitored upon depolarization of the cell membrane with the use of high K+ containing extracellular solutions. [Ca2+]i was measured in populations of cells as well as in individual cells using Fura-2, whereas the membrane potential (Em) was recorded in parallel experiments using patch-clamp techniques. Increasing the extracellular K+ concentration resulted in an instantaneous depolarization of Em of both FROB and NROB. This depolarization of Em did not significantly affect [Ca2+]i of populations of FROB and neonatal osteoblast precursors (NpROB). In contrast to FROB and NpROB, NROB populations responded to depolarization with significant transient [Ca2+]i increases that could be blocked by the calcium antagonist verapamil and were absent if extracellular Na+ was replaced for choline instead of K+. In individual cell measurements, response frequencies as well as the magnitude of [Ca2+]i responses upon depolarization of NROB were much higher than those of FROB, suggesting that more NROB than FROB possess VDCC. This phenomenon might point to a development-related expression of VDCC in the membranes of osteoblast-like cells.
Similar content being viewed by others
References
Ypey DL, Weidema AF, Höld KM, van der Laarse A, Ravesloot JH, van der Plas A, Nijweide PJ (1992) Voltage, calcium and stretch-activated ionic channels and intracellular calcium in bone cells. J Bone Miner Res (suppl 2) 7:S377–387
Herrmann-Erlee MPM, Gaillard PJ, Hekkelman JW, Nijweide PJ (1977) The effect of verapamil on the action of parathyroid hormone on embryonic bone in vitro. Eur J Pharmacol 46:51–58
Lerner U, Gustafson GT (1982) Inhibition of 1α-hydroxy-vitamin D3 stimulated bone resorption in tissue culture by calcium antagonist verapamil. Eur J Clin Invest 12:185–190
Ly SY, Rebut-Bonneton C, Miravet L (1985) Effect of the calcium antagonist diltiazem on in vitro and in vivo/in vitro bone resorption. Horm Metab Res 17:152–155
Guggino SE, Lajeunesse D, Wagner JA, Snyder SH (1989) Bone remodeling signaled by a dihydropyridine- and phenylalkylamine-sensitive calcium channel. Proc Natl Acad Sci USA 86: 2957–2960
Duriez J, Flautre B, Blary MC, Hardouin P (1993) Effects of the calcium channel blocker nifedipine on epiphyseal growth plate and bone turnover: a study in rabbit. Calcif Tissue Int 52:120–124
Kim YS, Yang IM, Kim SW, Kim JW, Kim KW, Choi YK (1991) Responses of osteoblastic cell line MC3T3-E1 cell to the calcium channel blocker diltiazem and verapamil. In: Morri H (ed). Calcium-regulating hormones II. Calcium transport, bone metabolism, and new drugs, vol 91. Karger, Basel, pp 43–49
Edelman A, Fritsch J, Balsan S (1986) Short-term effects of PTH on cultured rat osteoblasts: changes in membrane potential. Am J Physiol (Cell Physiol 20) 251:C483–490
Ferrier J, Ward A (1986) Electrophysiological differences between bone cell clones: membrane potential responses to parathyroid hormone and correlation with the cAMP response. J Cell Physiol 126:237–242
Yamaguchi DT, Hahn TJ, Iida-Klein A, Kleeman CR, Muallem S (1987) Parathyroid hormone-activated calcium channels in an osteoblast-like clonal osteosarcoma cell line. cAMP-dependent and cAMP-independent calcium channels. J Biol Chem 262: 7711–7718
Ferrier J, Ward-Kesthely A, Homble F, Ross S (1987) Further analysis of spontaneous membrane potential activity and the hyperpolarizing response to parathyroid hormone in osteoblast-like cells. J Cell Physiol 130:344–351
Ferrier J, Kesthely A, Xia S-L (1992) Hormone responses of in vitro bone nodule cells: studies on changes of intracellular calcium and membrane potential in response to parathyroid hormone and calcitonin. Bone Miner 19:103–116
Guggino SE, Wagner JA, Snowman AM, Hester LD, Sacktor B, Snyder SH (1988) Phenylalkylamine-sensitive calcium channels in osteoblast-like osteosarcoma cells. Characterization by ligand binding and single channel recordings. J Biol Chem 263: 10155–10161
Duncan R, Misler S (1989) Voltage-activated and stretch-activated Ba2+ conducting channels in an osteoblast-like cell line (UMR 106). FEBS Lett 251:17–21
Grygorczyk C, Grygorczyk R, Ferrier J (1989) Osteoblastic cells have L-type calcium channels. Bone Miner 7:137–148
Karpinski E, Wu L, Civitelli R, Avioli LV, Hruska KA, Pang PKT (1989) A dihydropyridine-sensitive calcium channel in rodent osteoblastic cells. Calcif Tissue Int 45:54–57
Chesnoy-Marchais D, Fritsch J (1988) Voltage-gated sodium and calcium currents in rat osteoblasts. J Physiol (Lond) 398: 291–311
Yamaguchi DT, Green J, Kleeman CR, Muallem S (1989) Properties of the depolarization-activated calcium and barium entry in osteoblast-like cells. J Biol Chem 264:197–204
Ravesloot JH, van Houten RJ, Ypey DL, Nijweide PJ (1990) Identification of Ca2+-activated K+ channels in cells of embryonic chick osteoblast cultures. J Bone Miner Res 5:1201–1210
Krieger NS (1992) Demonstration of sodium/calcium exchange in rodent osteoblasts. J Bone Miner Res 7:1105–1111
Eisner DA, Lederer WJ (1985) Na−Ca exchange: stoichiometry and electrogenicity. Am J Physiol (Cell Physiol 17) 248:C189–202
Hamill OP, Marty A, neher E, Sakmann B, Sigworth B (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free patches. Pflügers Arch 391: 85–100
Guenther HL, Hofstetter W, Stutzer A, Mühlbauer R, Fleisch H (1989) Evidence for heterogeneity of the osteoblastic phenotype determined with clonal rat bone cells established from transforming growth factor-β-induced cell colonies grown anchorage independently in semisolid medium. Endocrinology 125:2092–2102
Wiltink A, Maassen van den Brink A, Herrmann-Erlee MPM, Van der Meer JM, Van der Plas A, Willems PHGM, Van Duijn B, Nijweide PJ, Ypey DL (1993) Heterogeneity of intracellular calcium responses to parathyroid hormone and thrombin in primary osteoblast-like cells and UMR106-01 cells: correlations with culture conditions, intracellular calcium concentration and differentiation state. Cell Calcium 14:591–600
Herrmann-Erlee MPM, van der Meer JM, Löwik CWGM, Van Leeuwen JPTM, Boonekamp PM (1988) Different roles for calcium and cyclic AMP in the action of PTH: studies in bone explants and isolated bone cells. Bone 9:93–100
Wong G (1990) Isolation and behavior of isolated bone-forming cells. In: Hall BK (ed) Bone: the osteoblast and osteocyte, vol. 1. Telford Press Inc, New Jersey, USA, pp 171–192
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of calcium indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Moore EDW, Becker PL, Fogarty KE, Williams DA, Fay FS (1990) Ca2+ imaging in single living cells: theoretical and practical issues. Cell Calcium 11:157–179
Wiltink A, van der Laarse A, Herrmann-Erlee MPM, van der Meer JM, Ypey DL (1993) The use of the fluorescent probe Fura-2 for intracellular free calcium measurements: some methodological aspects. In: Bach PH, Reynolds CH, Clark JM, Poole PL, Mottley J (eds) Biotechnology applications of microinjection, microscopic imaging and fluorescence. Plenum Publishing Co, New York, USA, pp 131–140
Nitschke R, Fröbe U, Greger R (1991) Antidiuretic hormone acts via V1 receptors on intracellular calcium in the isolated perfused rabbit cortical thick ascending limb. Pflügers Arch 417 622–632
Xia S-L, Ferrier J (1992) Propagation of a calcium pulse between osteoblastic cells. Biochem Biophys Res Comm 186: 1212–1219
Schirrmacher K, Schmitz I, Winterhager E, Traub O, Brümmer F, Jones D, Bingmann D (1992) Characterization of gap junctions between osteoblast-like cells in culture. Calcif Tissue Int 51:285–290
Ypey DL, Clapham DE (1984) Development of a delayed outward-rectifying K+ conductance in cultured mouse peritoneal macrophages. Proc Natl Acad Sci USA 81:3083–3087
Gotti C, Sher E, Cabrini D, Bondiolotti G, Wanke E, Mancinelli E, Clementi F (1987) Cholinergic receptors, ion channels, neurotransmitter synthesis, and neurite outgrowth are independently regulated during the in vitro differentiation of a human neuroblastoma cell line. Differentiation 34:144–155
Lewis RS, Cahalan MD (1988) Subset-specific expression of potassium channels in developing murine T lymphocytes. Science 239:771–775
Hall ZW, Sanes JR (1993) Synaptic structure and development: the neuromuscular junction. Cell 72:99–121
Berridge MJ (1993) Inositol trisphosphate and calcium signalling. Nature (Lond) 361:315–325
Fox J (1988) Verapamil induces PTH resistance but increases duodenal calcium absorption in rats. Am J Physiol (Endocrinol Metab 18) 255:E702–707
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wiltink, A., Van Duijn, B., Weidema, A.F. et al. Differential depolarization-activated calcium responses in fetal and neonatal rat osteoblast-like cells. Calcif Tissue Int 54, 278–283 (1994). https://doi.org/10.1007/BF00295951
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00295951