Abstract
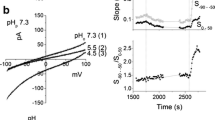
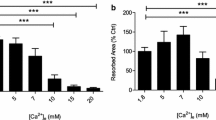
Osteoclasts are highly differentiated bone-resorbing cells and play a significant role in bone remodelling. In the resorption pit, inorganic phosphate (Pi) concentrations increase because of degradation of hydroxyapatite. We studied effects of extracellular Pi on voltage-gated H+ channels in osteoclast-like cells derived from a macrophage cell line (RAW264). Extracellular Pi (1.25–20 mM) increased the H+ channel currents dose dependently and reversibly. The Pi-induced increases were attenuated by removal of extracellular Na+ and by phosphonoformic acid, a blocker of Na+-dependent Pi transporters. Pi increased the maximal conductance, decreased activation time constant, increased deactivation time constant, and shifted the conductance-voltage relationship to more negative voltages. The most marked change was enhanced gating which was mainly caused by elevation of intracellular Pi levels. The Pi-induced enhanced gating was partially inhibited by protein kinase C (PKC) inhibitors, GF109203X and staurosporine, indicating that PKC-mediated phosphorylation was involved in part. The increase in the maximal conductance was mainly due to accompanying decrease in intracellular pH. These effects of Pi were not affected by intracellular Mg2+, bafilomycin A1 (V-ATPase inhibitor) and removal of intracellular ATP. Extracellular Pi also upregulated reactive oxygen species (ROS). Diphenyleneiodonium chloride, an inhibitor of NADPH oxidases, decreased ROS production and partially attenuated the enhanced gating. In the cells during later passages where osteoclastogenesis declined, H+ channel activities and ROS production were both modest. These results suggest that, in osteoclasts, ambient Pi is a common enhancer for H+ channels and ROS production and that potentiation of H+ channels may help ROS production.






Similar content being viewed by others
References
Andrini O, Meinild AK, Ghezzi C, Murer H, Foster IC (2012) Lithium interactions with Na+-coupled inorganic phosphate cotransporters: insights into the mechanism of sequential cation binding. Am J Phys 302:C539–C554. doi:10.1152/ajpcell.00364.2011
Babior BM (1999) NADPH oxidase: an update. Blood 93:1464–1476
Bánfi B, Schrenzel J, Nüsse O, Lew DP, Ligeti E, Krause KH, Demaurex N (1999) A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J Exp Med 190:183–194. doi:10.1084/jem.190.2.183
Bergwitz C, Jüppner H (2011) Phosphate sensing. Adv Chronic Kidney Dis 18:132–144. doi:10.1053/j.ackd.2011.01.004
Bevington A, Kemp GJ, Graham R, Russell G (1992) Phosphate-sensitive enzymes: a possible molecular basis for cellular disorders of phosphate metabolism. Clin Chem Enzym Comms 4:235–257
Bevington A, Mundy KI, Yates AJP, Kanis JA, Russell RGG, Taylor DJ, Rajagopalan B, Radda GK (1986) A study of intracellular orthophosphate concentration in human muscle and erythrocytes by 31P nuclear magnetic resonance spectroscopy and selective chemical assay. Clin Sci 71:729–735. doi:10.1042/cs0710729
Busch A, Waldegger S, Herzer T, Biber J, Markovich D, Hayes G, Murer H, Lang F (1994) Electrophysiological analysis of Na+/Pi contransport mediated by a transporter cloned from rat kidney and expressed in Xenopus oocytes. Proc Natl Acid Sci USA 91:8205–8208
DeCoursey TE (2013) Voltage-gated proton channels: molecular biology, physiology, and pathophysiology of the Hv family. Physiol Rev 93:599–652. doi:10.1152/physrev.00011.2012
DeCoursey TE, Cherny VV, Zhou W, Thomas LL (2000) Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc Natl Acad Sci U S A 97:6885–6889. doi:10.1073/pnas.100047297
Di Marco GS, Hausberg M, Hillebrand U, Rustemeyer P, Wittkowski W, Lang D, Pavenstӓdt H (2008) Increased inorganic phosphate induces human endothelial cell apoptosis in vitro. Am J Phys 294:F1381–F1387. doi:10.1152/ajprenal.00003.2008
Gupta A, Guo X-L, Alvarez UM, Hruska KA (1997) Regulation of sodium-dependent phosphate transport in osteoclasts. J Clin Invest 100:538–549. doi:10.1172/JCI119563
Gupta A, Tenenhouse HS, Hoag HM, Wang D, Khadeer MA, Namba N, Feng X, Hruska KA (2001) Identification of the type II Na+-Pi cotransporter (Npt2) in the osteoclast and the skeletal phenotype of Npt2−/− mice. Bone 29:467–476
Henderson LM, Chappell JB, Jones OTG (1988) Internal pH changes associated with the activity of NADPH oxidase of human neutrophils: further evidence for the presence of an H+ conducting channel. Biochem J 251:563–567. doi:10.1042/bj2510563
Hong L, Pathak MM, Kim IH, Ta D, Tombola F (2013) Voltage-sensing domain of voltage-gated proton channel Hv1 shares mechanism of block with pore domains. Neuron 77:274–287. doi:10.1016/j.neuron.2012.11.013
Humez S, Fournier F, Guilbault P (1995) A voltage-dependent and pH-sensitive proton current in Rana esculenta oocytes. J Memb Biol 147:207–215
Ito M, Haito S, Furumoto M, Uehata Y, Sakurai A, Segawa H, Tatsumi S, Kuwahata M, Miyamoto K (2007) Unique uptake and efflux systems of inorganic phosphate in osteoclast-like cells. Am J Phys 292:C526–C534. doi:10.1152/ajpcell.00357.2006
Ito M, Matsuka N, Izuka M, Haito S, Sakai Y, Nakamura R, Segawa H, Kuwahata M, Yamamoto H, Pike WJ, Miyamoto K (2005) Characterization of inorganic phosphate transport in osteoclast-like cells. Am J Phys 288:C921–C931. doi:10.1152/ajpcell.00412.2004
Kanatani M, Sugimoto T, Kano J, Kanzawa M, Chihara K (2003) Effect of high phosphate concentration on osteoclast differentiation as well as bone resorbing activity. J Cell Physiol 196:180–189. doi:10.1002/jcp.10270
Khadeer MA, Tang Z, Tenenhouse HS, Eiden MV, Murer H, Hernando N, Weinman EJ, Chellaiah MA, Gupta A (2003) Na+-dependent phosphate transporters in the murine osteoclast: cellular distribution and protein interactions. Am J Physiol 284:C1633–C1644. doi:10.1152/ajpcell.00580.2002
Kuno M, Ando H, Morihata H, Sakai H, Mori H, Sawada M, Oiki S (2009) Temperature dependence of proton permeation through a voltage-gated proton channel. J Gen Physiol 134:191–205. doi:10.1085/jgp.200910213
Kuno M, Li G, Moriura Y, Hino Y, Kawawaki J, Sakai H (2016) Acid-inducible proton influx currents in the plasma membrane of murine osteoclast-like cells. Pflűgers Archiv – Eur. J Physiol 468:837–847. doi:10.1007/s00424-016-1796-7
Laver DR, Lenz GKE, Dulhunty AF (2001) Phosphate ion channels in sarcoplasmic reticulum of rabbit skeletal muscle. J Physiol 535:715–728
Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, Kim N, Lee SY (2005) A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 106:852–859. doi:10.1182/blood-2004-09-3662
Manolagas SC (2010) From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31:266–300. doi:10.1210/er.2009-0024
Meijles DN, Fan LM, Howlin BJ, Li J-M (2014) Molecular insights of p47phox phosphorylation dynamics in the regulation of NADPH oxidase activation and superoxide production. J Biol Chem 289:22759–22770. doi:10.1074/jbc.M114.561159
Michigami T (2013) Extracellular phosphate as a signaling molecule. In: Razzaque MS (ed) Phosphate and vitamin D in chronic kidney disease. Contrib Nephrol. Basel, Karger, vol 180, pp14–24. doi: 10.1159/000346776
Morgan D, Cherny VV, Finnegan A, Bollinger J, Gelb MH, DeCousey TE (2007) Sustained activation of proton channels and NADPH oxidase in human eosinophils and murine granulocytes requires PKC but not cPLA2α activity. J Physiol 579:327–344. doi:10.1113/jphysiol.2006.124248
Mori H, Sakai H, Morihata H, Kawawaki J, Amano H, Yamano T, Kuno M (2003) Regulatory mechanisms and physiological relevance of a voltage-gated H+ channel in murine osteoclasts: phorbol myristate acetate induces cell acidosis and the channel activation. J Bone Mine Res 18:2069–2076
Mozar A, Haren N, Chasseraud M, Louvet L, Mazière C, Wattel A, Mentaverri R, Morlière P, Kamel S, Brazier M, Mazière JC, Massy ZA (2008) High extracellular inorganic phosphate concentration inhibits RANK-RANKL signaling in osteoclast-like cells. J Cell Physiol 215:47–54. doi:10.1002/jcp.21283
Musset B, Capasso M, Cherny VV, Morgan D, Bhamrah M, Dyer MJS, DeCoursey TE (2010) Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes. J Biol Chem 285:5117–5121. doi:10.1074/jbc.C109.082727
Musset B, Cherny VV, Morgan D, DeCoursey TE (2009) The intimate and mysterious relationship between proton channels and NADPH oxidase. FEBS Lett 583:7–12. doi:10.1016/j.febslet.2008.12.005
Nordström T, Rotstein OD, Romanek R, Asotra S, Heersche JNM, Manolson MF, Brisseau GF, Grinstein S (1995) Regulation of cytoplasmic pH in osteoclasts: contribution of proton pumps and a proton-selective conductance. J Biol Chem 270:2203–2212. doi:10.1074/jbc.270.5.2203
Perisic O, Wilson MI, Karathanassis D, Bravo J, Pacold ME, Ellson CD, Hawkins PT, Stephens L, Williams RL (2004) The role of phosphoinositides and phosphorylation in regulation of NADPH oxidase. Adv Enzym Regul 44:279–298. doi:10.1016/j.advenzreg.2003.11.003
Sakai H, Kawawaki J, Moriura Y, Mori H, Morihata H, Kuno M (2006) pH dependence and inhibition by extracellular calcium of proton currents via plasmalemmal vacuolar-type H+-ATPase in murine osteoclasts. J Physiol 576:417–425. doi:10.1113/jphysiol.2006.117176
Sakai H, Li G, Hino Y, Moriura Y, Kawawaki J, Sawada M, Kuno M (2013) Increases in intracellular pH facilitate endocytosis and decrease availability of voltage-gated proton channels in osteoclasts and microglia. J Physiol 591:5851–5866. doi:10.1113/jphysiol.2013.263558
Silver IA, Murrills RJ, Etherington DJ (1988) Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res 175:266–276
Yates AJ, Oreffo ROC, Mayor K, Mundy GR (1991) Inhibition of bone resorption by inorganic phosphate is mediated by both reduced osteoclast formation and decreased activity of mature osteoclasts. J Bone Miner Res 6:473–478. doi:10.1002/jbmr.5650060508
Acknowledgments
We thank Y. Hino, Y. Moriura and J. Kawawaki for technical assistance. This work was supported by JSPS KAKENHI Grant Number JP15K08184.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Fig. S1
PMA-induced ROS production in RAW-derived osteoclasts. The time courses of ROS production in control (squares) and after addition of PMA (phorbol 12-myristate 13-acetate) (circles). Triangles are data obtained in the presence of PMA and ZnCl2. The cells (0.3 × 105 cells) were incubated with L012 for 8 min before the recordings. The data represent the average of triplicate measurements of chemiluminescence intensities, expressed as relative to the values at the onset of recordings in a batch. PMA; 200 nM. ZnCl2; 200 μM. *p < 0.05; the PMA-induced ROS production with and without ZnCl2 are compared. (PDF 14 kb)
Rights and permissions
About this article
Cite this article
Li, G., Miura, K. & Kuno, M. Extracellular phosphates enhance activities of voltage-gated proton channels and production of reactive oxygen species in murine osteoclast-like cells. Pflugers Arch - Eur J Physiol 469, 279–292 (2017). https://doi.org/10.1007/s00424-016-1931-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-016-1931-5