Summary
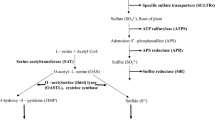
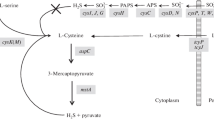
cysA− mutants of Aspergillus nidulans lacking serine transacetylase were isolated. cysA− mutants are cysteine auxotrophs only when a second mutation either blocking cystathionine β-synthase (mecA−) or γ-cystathionase (mecB−) is present. These findings prove that homocysteine originating from O-acetylhomoserine and sulfide can be converted not only to methionine, but also to cysteine via the reverse trans-sulfurylation pathway.
Similar content being viewed by others
References
Arst, H. N., Jr.: Genetic analysis of the first steps of sulphate metabolism in Aspergillus nidulans. Nature (Lond.) 219, 268–270 (1968)
Giovanelli, J., Mudd, S. H.: Transsulfuration in higher plants. Partial purification and properties of γ-cystathionase of spinach. Biochim. biophys. Acta (Amst.) 227, 654–670 (1971)
Kredich, N. M., Tomkins, G. M.: The enzyme synthesis of L-cysteine in E. coli and S. typhimurium. J. biol. Chem. 241, 4955–4965 (1966)
Paszewski, A., Grabski, J.: Studies on β-cystathionase and O-acetylhomoserine sulfhydrylase as the enzymes of alternative methionine biosynthetic pathways in Aspergillus nidulans. Acta biochim. pol. 20, 159–168 (1973)
Pieniążek, N. J., Kowalska, I., Stępień, P. P.: Deficiency in methionine adenosyltransferase resulting in limited repressibility of methionine biosynthetic enzymes in Aspergillus nidulans. Molec. gen. Genet. 126, 367–374 (1973)
Pieniążek, N. J., Stępień, P. P., Paszewski, A.: An Aspergillus nidulans mutant lacking c cystathionine β-synthase: identity of L-serine sulfhydrylase with cytathionine β-synthase and its distinctness from O-acetyl-L-serine sulfhydrylase. Biochim. biophys. Acta (Amst.) 297, 37–47 (1973)
Savin, M. A., Flavin, M., Slaugther, C.: Regulation of homocysteine biosynthesis in Salmonella typhimurium. J. Bact. 111, 547–556 (1972)
Smith, I. K., Thompson, J. F.: Utilisation of S-methylcysteine and methylmercaptan by methionineless mutants of Neurospora and the pathway of their conversion to methionine. II. Enzyme studies. Biochim. biophys. Acta (Amst.) 184, 130–138 (1969)
Wiebers, J. L., Garner, H. R.: Acyl derivatives of homoserine as substrates for homocysteine synthesis in Neurospora crassa, yeast and Escherichia coli. J. Biol. Chem. 42, 5644–5649 (1967)
Author information
Authors and Affiliations
Additional information
Communicated by F. Kaudewitz
Rights and permissions
About this article
Cite this article
Pieniążek, N.J., Bal, J., Balbin, E. et al. An Aspergillus nidulans mutant lacking serine transacetylase: Evidence for two pathways of cysteine biosynthesis. Molec. Gen. Genet. 132, 363–366 (1974). https://doi.org/10.1007/BF00268575
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00268575