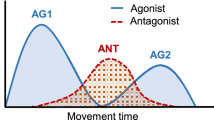
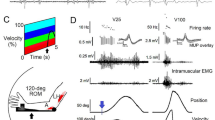
Summary
In rapid goal-directed elbow flexion movements the influence of both movement amplitude and inertial load on the three-burst pattern and the consequences on movement time were studied. Subjects performed visually guided, self-paced movements as rapidly and as accurately as possible. An increase of both the movement amplitude and the inertial load were found to be interacting factors for the modulation of the three-burst-pattern and movement time. The first biceps burst progressively increased in duration and amplitude for larger movements, resulting in prolonged movement times. Surplus inertial loads further prolonged the agonist burst for large, but not for small movement amplitudes. The activity of the antagonist burst, in contrast, was largest in small movements and successively decreased at increasing movement amplitudes. Its duration, however, remained fairly constant. As was similarly observed for the agonist burst, surplus inertial loads lead to a prolongation of antagonist burst duration and an increase of the activity integral for large, but not for small movement amplitudes. It is suggested that in elbow flexion movements the programming of fastest goal-directed movements must take into account neural constraints and biomechanical characteristics of the agonist muscle and the antagonist muscle. Due to neural constraints of the biceps muscle, in contrast to finger movements, the concept of movement time invariance does not hold for elbow movements. Furthermore, neural constraints of the antagonist muscle lead to a limited force production of the agonist muscle at small movement amplitudes in order to avoid an overload of the braking process. The complexity of the relationship between neural and mechanical factors indicate that the size and timing of the three-burst-pattern has to be subtly adjusted to the precise nature of the task and its biomechanical characteristics.
Similar content being viewed by others
References
Adam JER, Hallett M, Marsden CD, Merton PA, Morton HB (1976) Absence of the first component of the long-latency human stretch reflex in a thumb muscle when it is used as an antagonist. J Physiol (Lond) 260: 67–68
Angel RW (1974) Electromyography during voluntary movement: the two-burst pattern. EEG Clin Neurophysiol 36: 493–498
Berardelli A, Rothwell JC, Day BL, Kachi T, Marsden CD (1984) Duration of the first agonist EMG-burst in ballistic arm movements. Brain Res 304: 183–187
Brown SHC, Cooke JD (1981) Amplitude- and instruction-dependent modulation of movement-related electromyogram activity in humans. J Physiol (Lond) 316: 97–107
Brown SHC, Cooke JD (1984) Initial agonist burst duration depends on movement amplitude. Exp Brain Res 55: 523–527
Cooke JD (1980) The organisation of simple, skilled movements. In: Stelmach A, Requin J (eds) Tutorials in motor behaviour. North-Holland, Amsterdam, pp 199–212
Day BL, Marsden CD (1982) Accurate repositioning of the human thumb against unpredictable dynamic loads is dependent upon peripheral feed-back. J Physiol (Lond) 327: 393–407
Day BL, Rothwell JC, Marsden CD (1983) Interaction between the long-latency stretch reflex and voluntary electro-myographic activity prior to a rapid voluntary motor reaction. Brain Res 270: 55–62
Freund HJ, Büdingen HJ (1978) The relationship between speed and amplitude of the fastest voluntary contractions of human arm muscles. Exp Brain Res 31: 1–12
Hallett M, Shahani BT, Young RR (1975) EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiat 38: 1154–1162
Hallett M, Marsden CD (1979) Ballistic flexion movements of the human thumb. J Physiol (Lond) 294: 33–50
Hopf HC, Lowitzsch K, Schlegel HJ (1973) Central versus proprioceptive influences in brisk voluntary movements. In: Desmedt JE (ed) New developments in EMG and clinical neurophysiology, Vol III. Karger, Basel, pp 273–276
Hufschmidt H-J (1952) Die rasche Willkürkontraktion. Z Biol 107: 1–24
Lestienne F (1979). Effects of inertial load and velocity on the braking process of voluntary limb movements. Exp Brain Res 35: 407–418
Marsden CD, Merton PA, Morton HB (1976) Servo action in the human thumb. J Physiol (Lond) 257: 1–44
Marsden CD, Merton PA, Morton HB, Adam J (1978) The effect of lesions of the central nervous system on long-latency stretch reflexes in the human thumb. In: Desmedt JE (ed) Progress in clinical neurophysiology, Vol 4: Cerebral motor control in man: long loop mechanisms. Karger, Basel, pp 334–341
Marsden JA, Obeso A, Rothwell JC (1983) The function of the antagonist muscle during fast limb movements in man. J Physiol (Lond) 335: 1–13
Meinck H-M, Benecke R, Meyer W, Höhne J, Conrad B (1984) Human ballistic finger flexion: uncoupling of the three-burst pattern. Exp Brain Res 55: 127–133
Mortimer SA, Webster DD, Duckich TG (1981) Changes in short and long-latency stretch responses during the transition from posture to movement. Brain Res 229: 337–351
Wacholder K (1923) Untersuchungen über die Innervation and Koordination der Bewegungen mit Hilfe der Aktionsströme. II. Die Koordination der Agonisten und Antagonisten bei den menschlichen Bewegungen. Pflügers Arch Ges Physiol 199: 625–650
Author information
Authors and Affiliations
Additional information
Supported by the Deutsche Forschungsgemeinschaft (SFB 33)
Rights and permissions
About this article
Cite this article
Benecke, R., Meinck, H.M. & Conrad, B. Rapid goal-directed elbow flexion movements: limitations of the speed control system due to neural constraints. Exp Brain Res 59, 470–477 (1985). https://doi.org/10.1007/BF00261336
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00261336