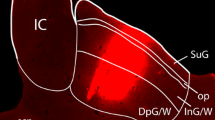
Summary
Despite extensive behavioural work on the rat superior colliculus, its descending efferent pathways have not been fully characterised with modern anatomical tract-tracing techniques. To investigate these pathways, wheatgerm-agglutinin conjugated with horseradish peroxidase (1%) was injected at various locations within the superior colliculus of hooded rats. Label judged to be transported orthogradely was plotted on coronal sections modified from the atlas of Paxinos and Watson (1982). Two major descending pathways were identified, (i) The bulk of the fibres in the ipsilateral descending pathway leave the superior colliculus ventrolaterally, and course around the lateral margin of the midbrain reticular formation. Caudally, projecting fibres leave the main bundle to innervate the cuneiform nucleus, and parts of the pontomedullary reticular formation. Terminal fields associated with the major bundle of fibres are found in an area medial to the brachium of the inferior colliculus; the parabigeminal nucleus and adjacent tegmentum; the ventrolateral midbrain reticular formation; and the lateral pontine nuclei, (ii) The fibres of the main contralateral descending pathway leave the superior colliculus ventromedially, to cross midline in the dorsal tegmental decussation. They immediately turn caudally to join the predorsal bundle, in which they run the length of the brainstem to reach the cervical spinal cord. Major terminal fields occur in nucleus reticularis tegmenti pontis; the pedunculopontine/ parabrachial area; paramedian pontomedullary reticular formation; and inferior olive. In addition there is lighter labelling in many areas of the pontomedullary reticular formation and in the cervical spinal cord. There was also a much sparser contralateral descending projection that crossed midline in the tectal commissure, and sent terminals to the contralateral cuneiform area and adjoining regions. These results suggest that the distribution of the descending efferent pathways from the superior colliculus in rats is similar to those described in other species. The fact that the two major pathways project to quite different terminal areas, together with previous findings that they have separate cells of origin within the tectum, suggests that they may also be functionally distinct.
Similar content being viewed by others
References
Akaike T (1985) Electrophysiological analysis of the tectoolivocerebellar (lobule VII) projection in the rat. Brain Res 340: 369–372
Brodal P, Dietrichs E, Bjaalie JG, Nordby T, Walberg F (1983) Is lectin-coupled horseradish peroxidase taken up and transported by undamaged as well as damaged fibers in the central nervous system? Brain Res 278: 1–9
Burne RA, Azizi SA, Mihailoff GA, Woodward DJ (1983) The tectopontine projection in the rat with comments on visual pathways to the basilar pons. J Comp Neurol 202: 287–307
Chevalier G, Deniau JM (1984) Spatio-temporal organization of a branched tecto-spinal/tecto-diencephalic neuronal system. Neuroscience 12: 427–439
Cools AR, Coolen JMM, Smit JC, Ellenbroek BA (1984) The striato-nigro-collicular pathway and explosive running behaviour: functional interaction between neostriatal dopamine and collicular GABA. Eur J Pharmacol 100: 71–78
Cools AR, Ellenbroek BA, van der Heuvel C (1983) Picrotoxin microinjections into the brain: a model of abrupt withdrawal ‘jumping’ behaviour in rats not exposed to any opiate? Eur J Pharmacol 90: 237–243
Dean P (1981) Visual pathways and acuity in hooded rats. Behav Brain Res 3: 239–271
Dean P, Mitchell IJ, Redgrave P (1986a) Head movements produced by microinjection of glutamate into superior colliculus of rats: evidence for involvement of multiple output pathways. Soc Neurosci Abstr 12: 1030
Dean P, Redgrave P (1984a) The superior colliculus and visual neglect in rat and hamster. I. Behavioural evidence. Brain Res Rev 8: 129–141
Dean P, Redgrave P (1984b) The superior colliculus and visual neglect in rat and hamster. II. Possible mechanisms. Brain Res Rev 8: 143–153
Dean P, Redgrave P (1984c) The superior colliculus and visual neglect in rat and hamster. III. Functional implications. Brain Res Rev 8: 155–163
Dean P, Redgrave P, Sahibzada N, Tsuji K (1986b) Head and body movements produced by electrical stimulation of superior colliculus in rats: effects of interruption of crossed tectoreticulospinal pathway. Neuroscience 19: 367–380
Dreher B, Sefton AJ, Ni SYK, Nisbett G (1985) The morphology, number, distribution and central projections of class I retinal ganglion cells in albino and hooded rats. Brain Behav Evol 26: 10–48
Edwards SB (1980) The deep cells of the superior colliculus: their reticular characteristics and structural organization. In: Hobson JA, Brazier MAB (eds) The reticular formation revisited. Raven Press, New York, pp 193–209
Edwards SB, Henkel CK (1978) Superior colliculus connections with the extraocular motor nuclei in the cat. J Comp Neurol 179: 451–468
Flumerfelt BA, Hrycyshyn AW (1985) Precerebellar nuclei and red nucleus. In: Paxinos G (ed) The rat ervous system, Vol 2. Academic Press, Sydney, pp 221–250
Gerfen CR, Staines WA, Arbuthnott GW, Fibiger HC (1982) Crossed connections of the substantia nigra in the rat. J Comp Neurol 207: 283–303
Gibson RA, Hansma DI, Houk JC, Robinson FR (1984) A sensitive low artifact TMB procedure for the demonstration of WGA-HRP in the CNS. Brain Res 298: 235–241
Glickstein M, May JG III (1982) Visual control of movement: the circuits which link visual to motor areas of the brain with special reference to the visual input to the pons and cerebellum. In: Neff WD (ed) Contributions to sensory physiology, Vol 7. Academic Press, New York, pp 103–145
Grantyn A, Grantyn R (1982) Axonal pattern and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp Brain Res 46: 243–256
Harting JK (1977) Descending pathways from the superior colliculus: an autoradiographic analysis in the rhesus monkey (Macaca mulatta). J Comp Neurol 173: 583–612
Harting JK, Hall WC, Diamond IT, Martin GF (1973) Anterograde degeneration study of the superior colliculus in Tupaia glis: evidence for a subdivision between the superficial and deep layers. J Comp Neurol 148: 361–386
Harting JK, Huerta MF (1984) The mammalian superior colliculus: studies of its morphology and connections. In: Vanegas H (ed) The comparative neurology of the optic tectum. Plenum Press, New York, pp 687–773
Henkel CK, Edwards SB (1978) The superior colliculus control of pinna movements in the cat: possible anatomical connections. J Comp Neurol 182: 763–776
Holstege G, Collewijn H (1982) The efferent connections of the nucleus of the optic tract and the superior colliculus in the rabbit. J Comp Neurol 209: 139–175
Huerta MF, Harting JK (1982) Tectal control of spinal cord activity: neuroanatomical demonstration of pathway connecting the superior colliculus with the cervical spinal cord grey. In: Kuypers HGJM, Martin GF (eds) Descending pathways to the spinal cord. Progress in brain res, Vol 57. Eisevier, Amsterdam, pp 293–328
Keay KA, Redgrave P, Dean P (1986) Changes in blood pressure and respiration elicited by electrical and chemical stimulation of the superior colliculus in rats. Neurosci Lett Suppl 26: S238
Kilpatrick IC, Collingridge GL, Starr MS (1982) Evidence for the participation of gamma-aminobutyrate containing neurons in striatal and nigral-derived circling in the rat. Neuroscience 7: 207–222
Künzle H, Schnyder H (1984) The isthmus-tegmentum complex in the turtle and rat: a comparative analysis of its interconnections with the optic tectum. Exp Brain Res 56: 509–522
Linden R, Perry VH (1983a) Massive retinotectal projection in rats. Brain Res 272: 145–149
Linden R, Perry VH (1983b) Retrograde and anterograde-transneuronal degeneration in the parabigeminal nucleus following tectal lesions in developing rats. J Comp Neurol 218: 270–281
May PJ, Vidal P-P, Baker R (1985) Electrophysiology of the paralemniscal-facial pathway. Soc Neurosci Abstr 11: 82
Mesulam M-M (1982) Principles of horseradish peroxidase neurohistochemistry and their applications for tracing neural pathways — axonal transport, enzyme histochemistry, and light microscopic analysis. In: Mesulam M-M (ed) Tracing neural connections with horseradish peroxidase. John Wiley, Chichester, pp 1–151
Miliaresis E, Philippe L (1984) The pontine substrate of circling behavior. Brain Res 293: 143–152
Mitchell IJ, Redgrave P, Dean P (1987) Potentiation of glutamateelicited defensive responses from tecto-recipient zone of cuneiform area in rat. Neurosci Lett Suppl 29: S 127
Olucha F, Martínez-García F, López-Garcia C (1985) A new stabilizing agent for the tetramethyl benzidine (TBM) reaction product in the histochemical detection of horseradish peroxidase (HRP). J Neurosci Meth 13: 131–138
Otani K, Kimura M, Tanaka K (1981) The laminally differentiated projections of the superior colliculus in the rat. Neurosci Lett Suppl 6: S113
Otani K, Kimura M, Yamada J (1975) The tectofugal projections in the rat and cat. Proc. 10th Internat. Cong. Anat. Tokyo, p 183
Papez JW, Freeman GL (1930) Superior colliculi and their fibre connections in the rat. J Comp Neurol 51: 409–439
Parker SM, Sinnamon HM (1983) Forward locomotion elicited by electrical stimulation in the diencephalon and mesencephalon of the awake rat. Physiol Behav 31: 581–587
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic Press, Sydney
Perry VH (1980) A tectocortical visual pathway in the rat. Neuroscience 5: 915–927
Petrovický P (1976) Projections from the tectum mesencephali to the brain stem structures in the rat. Folia Morphol 24: 41–48
Redgrave P, Dean P (1985) Tonic desynchronisation of cortical electroencephalogram by electrical and chemical stimulation of superior colliculus and surrounding structures in urethaneanaesthetised rats. Neuroscience 16: 659–671
Redgrave P, Dean P, Souki W, Lewis G (1981) Gnawing and changes in reactivity produced by microinjections of picrotoxin into the superior colliculus of rats. Psychopharmacology 75: 198–203
Redgrave P, Mitchell IJ, Dean P (1986a) Stereotaxic mapping of efferent descending pathways of the superior colliculus in rat: an anterograde WGA-HRP study. Neurosci Lett Suppl 26: S349
Redgrave P, Odekunle A, Dean P (1986b) Tectal cells of origin of predorsal bundle in rat: location and segregation from ipsilateral descending pathway. Exp Brain Res 63: 279–293
Reese BE (1984) The projection from the superior colliculus to the dorsal lateral geniculate nucleus in the rat. Brain Res 305: 162–168
Robertson RT (1983) Efferents of the pretectal complex: separate populations of neurons project to lateral thalamus and to inferior olive. Brain Res 258: 91–95
Sahibzada N, Dean P, Redgrave P (1986) Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J Neurosci 6: 723–733
Sefton AJ, Dreher B (1985) Visual system. In: Paxinos G (ed) The rat nervous system, Vol 1. Forebrain and midbrain. Academic Press, Sydney, pp 169–221
Smythe BA, Weber JT, Huerta MF, Harting JK (1979) The organization of visual inputs to the inferior olivary complex. Soc Neurosci Abstr 5: 808
Swanson LW, Mogenson GJ, Gerfen CR, Robinson P (1984) Evidence for a projection from the lateral preoptic area and substantia innominata to the mesencephalic locomotor region in the rat. Brain Res 295: 161–178
Swenson RS, Castro AJ (1983) The afferent connections of the inferior olivary complex in rats: a study using the retrograde transport of horseradish proxidase. Am J Anat 166: 329–341
Torigoe Y, Blanks RHI, Precht W (1986a) Anatomical studies on the nucleus reticularis tegmenti pontis in the pigmented rat. I. Cytoarchitecture, topography, and cerebral cortical afferents. J Comp Neurol 243: 71–87
Torigoe Y, Blanks RHI, Precht W (1986b) Anatomical studies on the nucleus reticularis tegmenti pontis in the pigmented rat. II. Subcortical afferents demonstrated by the retrograde transport of horseradish peroxidase. J Comp Neurol 243: 88–105
Waldron HA, Gwyn DG (1969) Descending nerve tracts in the spinal cord of the rat. I. Fibres from the midbrain. J Comp Neurol 137: 143–154
Yeomans JS, Pearce R, Wen D, Hawkins RD (1984) Mapping midbrain sites for circling using current-frequency trade-off data. Physiol Behav 32: 287–294
Zemlan FP, Behbehani MM (1984) Afferent projections to the nucleus cuneiformis in the rat. Neurosci Lett 52: 103–109
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Redgrave, P., Mitchell, I.J. & Dean, P. Descending projections from the superior colliculus in rat: a study using orthograde transport of wheatgerm-agglutinin conjugated horseradish peroxidase. Exp Brain Res 68, 147–167 (1987). https://doi.org/10.1007/BF00255241
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00255241