Summary
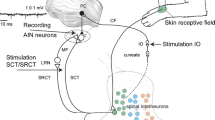
1. In anesthetized cats, we investigated excitatory and inhibitory inputs from the cerebral cortex to dentate nucleus neurons (DNNs) and determined the pathways responsible for mediating these inputs to DNNs. 2. Intracellular recordings were made from 201 DNNs whose locations were histologically determined. These neurons were identified as efferent DNNs by their antidromic responses to stimulation of the contralateral red nucleus (RN). Stimulation of the contralateral pericruciate cortex produced excitatory postsynaptic potentials (EPSPs) followed by long-lasting inhibitory postsynaptic potentials (IPSPs) in DNNs. The most effective stimulating sites for inducing these responses were observed in the medial portion (area 6) and its adjacent middle portion (area 4) of the precruciate gyrus. Convergence of cerebral inputs from area 4 and area 6 to single DNNs was rare. 3. To determine the precerebellar nuclei responsible for mediation of the cerebral inputs to the dentate nucleus (DN), we examined the effects of stimulation of the pontine nucleus (PN), the nucleus reticularis tegmenti pontis (NRTP) and the inferior olive (IO). Systematic mapping was made in the NRTP and the PN to find effective low-threshold stimulating sites for evoking monosynaptic EPSPs in DNNs. Stimulation of either the PN or the NRTP produced monosynaptic EPSPs and polysynaptic IPSPs in DNNs. Using a conditioning-testing paradigm (a conditioning stimulus to the cerebral peduncle (CP) and a test stimulus to the PN or the NRTP) and intracellular recordings from DNNs, we tested cerebral effects on neurons in the PN and the NRTP making a monosynaptic connection with DNNs. Conditioning stimulation of the CP facilitated PN- and NRTP-induced monosynaptic EPSPs in DNNs. This spatial facilitation indicated that the excitatory inputs from the cerebral cortex to DNNs are at least partly relayed via the PN and the NRTP. 4. Stimulation of the contralateral IO produced monosynaptic EPSPs and polysynaptic IPSPs in DNNs. These monosynaptic EPSPs were facilitated by conditioning stimulation of the CP, strongly suggesting that the IO is partly responsible for mediating excitatory inputs from the cerebral cortex to the DN. A comparison was made between the latencies of IO-evoked IPSPs in DNNs and the latencies of IO-evoked complex spikes in Purkinje cells. Such a comparison indicated that the shortest-latency IPSPs evoked from the IO were not mediated via the Purkinje cells and suggested the pathway mediated by inhibitory interneurons in the DN. 5. The functional significance of the excitatory inputs from the PN and the NRTP to the DN is discussed in relation to the motor control mechanisms of the cerebellum.
Similar content being viewed by others
References
Allen GI, Tsukahara N (1974) Cerebrocerebellar communication systems. Physiol Rev 54: 957–1006
Allen GI, Gilbert PFC, Yin TCT (1978) Convergence of cerebral inputs onto dentate neurons in monkey. Exp Brain Res 32: 151–170
Allen GI, Oshima T, Toyama K (1977a) The mode of synaptic linkage in the cerebro-ponto-cerebellar pathway investigated with intracellular recording from the pontine nuclei cells of the cat. Exp Brain Res 29: 123–136
Allen GI, Korn H, Oshima T, Toyama K (1975) The mode of synaptic linkage in the cerebro-ponto-cerebellar pathway of the cat. II. Responses of single cells in the pontine nuclei. Exp Brain Res 24: 15–36
Allen GI, Gilbert PFC, Marini R, Schultz W, Yin TCT (1977b) Integration of cerebral and peripheral inputs by interpositus neurons in monkey. Exp Brain Res 27: 81–99
Amatuni A, Tarnecki R, Wrobel A, Rajkowski J (1981) Interaction on extrocerebellar cortical inputs in dentate neurons of the cat. Acta Neurolobiol Exp 41: 373–390
Armstrong DM, Harvey RJ (1966) Responses in the inferior olive to stimulation of the cerebellar and cerebral cortices in the cat. J Physiol (Lond) 187: 553–574
Beitz AJ (1976) The topographical organization of the olivodentate and dentato-olivary pathways in the cat. Brain Res 115: 311–317
Bishop GA, McCrea RA, Kitai ST (1976) A horseradish peroxidase study of the cortico-olivary projection in the cat. Brain Res 116: 306–311
Brodal A (1981) Neurological anatomy in relation to clinical medicine. Oxford University Press, Oxford
Brodal A, Brodal P (1971) The organization of the nucleus reticularis tegmenti pontis in the cat in the light of experimental anatomical studies of its cerebral cortical afferents. Exp Brain Res 13: 90–110
Brodal A, Courville J (1973) Cerebellar corticonuclear projection in the cat. Crus II. An experimental study with silver methods. Brain Res 50: 1–23
Brodal A, Gogstad AC (1954) Rubro-cerebellar connections. An experimental study in the cat. Anat Rec 118: 455–486
Brodal A, Jansen J (1946) The ponto-cerebellar projection in the rabbit and cat. Experimental investigations. J Comp Neurol 84: 31–118
Brodal A, Szikla G (1972) The termination of the brachium conjunctivum descendens in the nucleus reticularis tegmenti pontis. An experimental anatomical study in the cat. Brain Res 39: 337–351
Brodal A, Destombes J, Lacerda AM, Angaut P (1972) A cerebellar projection onto the pontine nuclei. An experimental anatomical study in the cat. Exp Brain Res 16: 115–139
Brodal P (1968a) The corticopontine projection in the cat. I. Demonstration of a somatotopically organized projection from the primary sensorimotor cortex. Exp Brain Res 5: 212–237
Brodal P (1968b) The corticopontine projection in the cat. II. Demonstration of a somatotopically organized projection from the second somatosensory cortex. Arch Ital Biol 106: 310–332
Cajal S Ramony (1911) Histologie du system nerveux de l'homme et des vertébrés. Maloine, Paris
Chan-Palay V (1977) Cerebellar dentate nucleus, organization, cytology, and transmitters. Springer, Berlin Heidelberg New York
Courville J, Brodal A (1966) Rubrocerebellar connections in the cat. An experimental study with silver impregnation methods. J Comp Neurol 126: 471–485
Courville J, Augustine JR, Martel P (1977) Projections from the inferior olive to the cerebellar nuclei in the cat demonstrated by retrograde transport of horseradish peroxidase. Brain Res 130: 405–419
Dietrichs E, Bjaale JG, Brodal P (1983) Do pontocerebellar fibers send collaterals to the cerebellar nuclei? Brain Res 259: 127–131
Eccles JC (1964) The physiology of synapses. Springer, Berlin Heidelberg New York
Eccles JC, Ito M, Szentágothai J (1967) The cerebellum as a neuronal machine. Springer, Berlin Heidelberg New York
Eller T, Chan-Palay V (1976) Afferents to the cerebellar lateral nucleus. Evidence from retrograde transport of horseradish peroxidase after pressure injections through micropipettes. J Comp Neurol 166: 285–302
Futami T, Mitoma H, Shinoda Y (1984) Inputs from the cerebral cortex to dentate nucleus neurons in the cat. J Physiol Soc Jpn 46: 385
Futami T, Kano M, Sento S, Shinoda Y (1986) Synaptic organization of the cerebello-thalamo-cerebral pathway in the cat. III. Cerebellar input to corticofugal neurons destined for different subcortical nuclei in areas 4 and 6. Neurosci Res 3: 321–344
Groenewegen HJ, Voogd J, Freedman SL (1979) The parasagittal zonal organization within the olivocerebellar projection. II. Climbing fiber distribution in the intermediate and hemispheric parts of cat cerebellum. J Comp Neurol 183: 551–602
Hassler WR, Muhs-Clement K (1964) Architektonischer Aufbau des sensorimotorischen und parietalen Cortex der Katze. J Hirnforsch 6: 377–420
Hinman A, Carpenter MB (1959) Efferent fiber projections of the red nucleus in the cat. J Comp Neurol 113: 61–82
Hoddevik GH (1978) The projection from nucleus reticularis tegmenti pontis onto the cerebellum in the cat. A study using the methods of anterograde degeneration and retrograde axonal transport of horseradish peroxidase. Anat Embryol 153: 227–242
Ito M (1984) The cerebellum and neural control. Raven Press, New York
Ito M, Yoshida M (1966) The origin of cerebellar-induced inhibition of Deiters neurons. I. Monosynaptic initiation of the inhibitory postsynaptic potentials. Exp Brain Res 2: 330–349
Ito M, Yoshida M, Obata K, Kawai N, Udo M (1970) Inhibitory control of intracerebellar nuclei by the Purkinje cell axons. Exp Brain Res 10: 64–80
Jansen J, Brodal A (1954) Aspects of cerebellar anatomy. J Chr Grundersen, Oslo
Kitai ST, DeFrance JF, Hatada K, Kennedy DT (1974) Electrophysiological properties of lateral reticular nucleus cells. II. Synaptic activation. Exp Brain Res 21: 419–432
Kusama T, Otani K, Kawana E (1966) Projections of the motor, somatic sensory, auditory and visual cortices in cats. In: Tokizane T, Schadé JP (eds) Progress in brain research, Vol 21, Part A. Elsevier, Amsterdam, pp 292–322
Mano NI, Yamamoto KI (1980) Simple-spike activity of cerebellar Purkinje cells related to visually guided wrist tracking movement in the monkey. J Neurophysiol 43: 713–728
Matsushita M, Ikeda M (1970) Olivary projections to the cerebellar nuclei in the cat. Exp Brain Res 10: 488–500
Matsushita M, Ikeda M (1976) Projections from the lateral reticular nucleus to the cerebellar cortex and nuclei in the cat. Exp Brain Res 24: 403–421
Matsushita M, Iwahori N (1971) Structural organization of the interpositus and the dentate nuclei. Brain Res 35: 17–36
Mizuno N, Mochizuki K, Akimoto C, Matsushima R, Sasaki K (1973) Projections from the parietal cortex to the brain stem nuclei in the cat, with special reference to the parietal cerebrocerebellar system. J Comp Neurol 147: 511–522
Oka H, Jinnai K, Yamamoto T (1979) The parieto-rubro-olivary pathway in the cat. Exp Brain Res 37: 115–125
Oka H, Yoshida K, Yamamoto T, Samejima A (1985) Organization of afferent connections to the lateral and interpositus cerebellar nuclei from the brainstem nuclei: a horseradish peroxidase study in the cat. Neurosci Res 2: 321–333
Oka H, Sasaki K, Matsuda Y, Yasuda T, Mizuno N (1975) Responses of pontocerebellar neurones to stimulation of the parietal association and the frontal motor cortices. Brain Res 93: 399–407
Rüegg DG, Wiesendanger M (1975) Corticofugal effects from sensorimotor area I and somatosensory area II on neurones of the pontine nuclei in the cat. J Physiol (Lond) 247: 745–757
Saint-Cry JA, Courville J (1980) Projections from the motor cortex, midbrain, and vestibular nuclei to the inferior olive in the cat: anatomical organization and functional correlates. In: Courville J, de Montigny C, Lamarre Y (eds) The inferior olivary nucleus. Raven Press, New York, pp 97–124
Sasaki K, Strata P (1967) Responses evoked in the cerebellar cortex by stimulating mossy fiber pathways to the cerebellum. Exp Brain Res 3: 95–110
Sasaki K, Kawaguchi S, Shimono T, Yoneda Y (1969) Responses evoked in the cerebellar cortex by the pontine stimulation. Jpn J Physiol 19: 95–109
Schieber MH, Thach WT (1985) Trained slow tracking. II. Bidirectional discharge patterns of cerebellar nuclear, motor cortex, and spindle afferent neurons. J Neurophysiol 54: 1228–1270
Shinoda Y, Futami T, Kano M (1985) Synaptic organization of the cerebello-thalamo-cerebral pathway in the cat. II. Input-output organization of single thalamocortical neurons in the ventrolateral thalamus Neurosci Res 2: 157–180
Shinoda Y, Ohgaki T, Futami T (1986) The morphology of single lateral vestibulospinal tract axons in the lower cervical spinal cord of the cat. J Comp Neurol 249: 226–241
Shinoda Y, Yokota J, Futami T (1981) Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett 23: 7–12
Sousa-Pinto A (1969) Experimental anatomical demonstration of a cortico-olivary projection from area 6 (supplementary motor area?) in the cat. Brain Res 16: 73–83
Sousa-Pinto A, Brodal A (1969) Demonstration of a somatotopical pattern in the cortico-olivary projection in the cat. An experimental-anatomical study Exp Brain Res 8: 364–386
Thach WT (1968) Discharge of Purkinje and cerebellar neurons during rapidly alternating arm movement in the monkey. J Neurophysiol 31: 785–797
Tolbert DL, Massopust LC, Murphy MG, Young PA (1977) The anatomical organization of the cerebello-olivary projection in the cat. J Comp Neurol 170: 525–544
Voogd J (1964) The cerebellum of the cat. Structure and fibre connexions Proefschr Van Gorcum & Co NV, Assen
Voogd J (1969) The importance of fiber connections in the comparative anatomy of the mammalian cerebellum. In: Llinás R (ed) Neurobiology of cerebellar evolution and development. Amer Med Ass, Chicago, pp 493–514
Walberg F (1956) Descending connections to the inferior olive. J Comp Neurol 104: 77–173
Wetts R, Kalaska JF, Smith AM (1985) Cerebellar nuclear cell activity during antagonist cocontraction and reciprocal inhibition of forearm muscles. J Neurophysiol 54: 231–244
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shinoda, Y., Sugiuchi, Y. & Futami, T. Excitatory inputs to cerebellar dentate nucleus neurons from the cerebral cortex in the cat. Exp Brain Res 67, 299–315 (1987). https://doi.org/10.1007/BF00248551
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248551