Summary
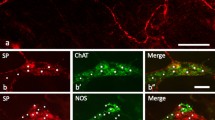
Peripheral nerve section or local capsaicin application produces depletion of substance P and an enzymatic marker, fluoride-resistant acid phosphatase (FRAP), from circumscribed regions of the terminal areas in the spinal cord. We have made use of this phenomenon to map the extent of central termination of subpopulations of primary afferent neurons containing substance P (SP), somatostatin (SOM), cholecystokinin (CCK), vasoactive intestinal polypeptide (VIP) and FRAP in the rat lumbar spinal cord following sciatic nerve section at midthigh level under ether anaesthesia. Between 2 days and 1 year postoperatively, the animals were perfused transcardially and SP, CCK, VIP and SOM were localised in frozen transverse sections of spinal cord segments L1 to S2 and their corresponding ganglia using unlabelled antibody immunohistochemistry. FRAP was localised using a modified Gomori method. SP, SOM, CCK and FRAP were maximally depleted from identical restricted areas of the dorsal horn of the third, fourth and fifth lumbar segments fifteen days after nerve section and remained so for a year. In contrast, VIP staining increased dramatically in the areas from which the other markers were depleted and showed the same time course. Moreover, a large number of neurons in the corresponding ganglia showed positive VIP immunoreactivity after axotomy but were absent from the unoperated side.
Similar content being viewed by others
References
Barbut D, Polak JM, Wall PD (1981) Substance P in the spinal cord dorsal horn decreases following peripheral nerve injury. Brain Res 205: 289–298
Bloom SR, Christofides ND, Delamarter J, Buell G, Kawashima E, Polak JM (1983) Diarrhoea in vipoma patients associated with cosecretion of a second active peptide (peptide histodine isoleucine) explained by single gene coding. Lancet ii: 1163–1165
Csillik B, Knyihar F (1978) Biodynamic plasticity in the Rolando substance. Prog Neurobiol 10: 203–230
Dalsgaard CJ, Vincent T, Hökfelt T, Lundberg JM, Dahlström A, Schultzberg M, Dockray GJ, Cuello AC (1982) Coexistence of cholecystokinin — and substance P-like peptides in the neurons of the dorsal root ganglia of the rat. Neurosci Lett 33:159–163
Dalsgaard CJ, Ygge J, Vincent SR, Ohrling M, Dockray GJ, Elde R (1984) Peripheral projections and neuropeptide coexistence in a subpopulation of fluoride-resistant acid phosphatase reactive spinal primary seasory neurons. Neurosci Lett 51: 139–144
Devor M, Claman D (1980) Mapping and plasticity of acid phosphatase afferents in the rat dorsal horn. Brain Res 190: 17–28
Gibson SJ, McGregor G, Bloom SR, Polak JM, Wall PD (1982) Local application of capsaicin to one sciatic nerve of the adult rat induces a marked depletion in the peptide content of the lumbar dorsal horn. Neuroscience 7: 3153–3162
Gibson SJ, Polak JM, Anand P, Blank MA, Morrison JFB, Kelly JS, Bloom SR (1984) The distribution and origin of VIP in the spinal cord of six mammalian species. Peptides (Fayetteville) 5: 201–207
Gibson SJ, Polak JM, Bloom SR, Wall PD (1981) The distribution of nine peptides in rat spinal cord with special emphasis on the substantia gelatinosa and on the area around the central canal (lamina X). J Comp Neurol 201: 65–79
Hökfelt T, Elde R, Johansson O, Luft R, Nilsson G, Arimura A (1976) Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience 1: 131–136
Honda CN, Rethelyi M, Petrusz P (1983) Preferential immunohistochemical localization of vasoactive intestinal polypeptide (VIP) in the sacral spinal cord of the cat: light and electron microscopic observations. J Neurosci 3: 2183–2196
Hunt SP, Rossi J (1985) Peptide- and non-peptide-containing unmyelinated primary afferents: the parallel processing of nociceptive information. Philos Trans R Soc Lond B308: 283–289
Itoh N, Obata K, Yanaihara N, Okamoto M (1983) Human preprovasoactive intestinal polypeptide contains a novel PHI-27-like peptide, PHM 27. Nature 304: 547–549
Jancso G, Hökfelt T, Lundberg JM, Kiraly E, Halasz N, Nilsson G, Terenius L, Rehfeld J, Steinbusch H, Verhofstad A, Elde R, Said E, Brown M (1981) Immunohistochemical studies on the effect of capsaicin on spinal and medullary peptide and monoamine neurons using antisera to substance P, gastrinCCK, somatostatin, VIP enkephalin, neurotensin and 5-hydroxytryptamine. J Neurocytol 10: 963–980
Kuo DC, Kawatani M, de Groat WC (1985) Vasoactive intestinal polypeptide identified in the thoracic dorsal root ganglia of the cat. Brain Res 330: 178–182
Larsson L-I, Rehfeld JF (1979) Localisation and molecular heterogeneity of cholecystokinin in the central and peripheral nervous system. Brain Res 165: 201–218
Ljungdahl A, Hökfelt T, Nilsson G (1978) Distribution of substance P-like immunoreactivity in the central nervous system of the rat I. Cell bodies and nerve terminals. Neuroscience 3: 861–943
Lundberg JM, Fahrenkrug J, Brimijoin S (1981) Characteristics of the axonal transport of vasoactive intestinal polypeptide (VIP) in the nerves of the cat. Acta Physiol Scand 112: 421–436
McGregor GP, Gibson SJ, Sabate IM, Blank MA, Christofides ND, Wall PD, Polak JM, Bloom SR (1984) Effect of peripheral nerve section and nerve crush on spinal cord neuropeptides in the rat: increased VIP and PHI in the dorsal horn. Neuroscience 13: 207–216
Nagy JI, Hunt SP (1982) Fluoride-resistant acid phosphatasecontaining neurons in dorsal root ganglia are separate from those containing substance P or somatostatin. Neuroscience 7: 89–97
Priestley JV, Bramwell S, Butcher LL, Cuello AC (1982) Effect of capsaicin on neuropeptides in areas of termination of primary sensory neurones. Neurochem Int 4: 57–65
Salt TE, Hill RH (1983) Neurotransmitter candidates of somatosensory primary afferent nerves. Neuroscience 10: 1083–1103
Sar M, Stumpf WE, Miller RJ, Chang K-J, Cuatrecasas P (1978) Immunohistochemical localization of enkephalin in rat brain and spinal cord. J Comp Neurol 182: 17–38
Shehab SAS, Atkinson ME (1984) Sciatic nerve section has variable effects on primary afferent neuropeptides. J Anat 139: 725
Shehab SAS, Atkinso ME (1986) Vasoactive intestinal polypeptide (VIP) increases after peripheral axotomy of the sciatic nerve originates from primary afferent neurons. Brain Res (in press)
Tuchscherer MM, Seybold VS (1985) Immunohistochemical studies of substance P, cholecystokinin-octapeptide and somatostatin in dorsal root ganglia of the rat. Neuroscience 14: 593–605
Yaksh TL, Enstaquo O, Abay EO, Go VLW (1982) Studies on the location and release of cholecystokinin and vasoactive intestinal peptide in rat and cat spinal cord. Brain Res 242: 279–290
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shehab, S.A.S., Atkinson, M.E. Vasoactive intestinal polypeptide increases in areas of the dorsal horn of the spinal cord from which other neuropeptides are depleted following peripheral axotomy. Exp Brain Res 62, 422–430 (1986). https://doi.org/10.1007/BF00238861
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00238861