Summary
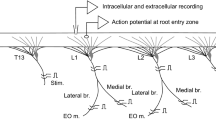
1. Interactions between phrenic motoneurons have been analysed in anaesthetized, paralyzed cats after C3 to C7 deafferentation. Effects of electrical stimulation of the C5 phrenic axons have been studied on thin filaments dissected from the stimulated nerve. Repetitive stimulation could elicit, after the primary direct response of the stimulated axons, a secondary response named Recurrent Response, RR. 2. RRs have been obtained in 117/186 phrenic axons. They appear sporadically (mean occurrence: 3.75 RRs elicited by 100 shocks of stimulation) at a constant latency. They originate from a spinal mechanism since they persist after C2 transection and disappear after section of the ventral roots. 3. The mechanism responsible for RR shows spatial and temporal facilitation. The RR probability increases with the number of antidromically invaded motoneurons as revealed by changes either of stimulation intensity or of central respiratory drive. However, RR could be evoked in a motoneuron without an antidromic volley in its axon. 4. Systemic injections of nicotinic blocking drugs such as dihydro-β-erytroidin or mecamylamine decrease or suppress the occurrence of RR; therefore, cholinergic synapses are involved in the RR generating process. 5. RR are assumed to be due to direct excitatory interactions between homonymous motoneurons. Recurrent axon collaterals impinging directly on neighbouring motoneurons would link together the different motoneurons of the phrenic pool. The functional significance of this phenomenon is discussed.
Similar content being viewed by others
References
Barillot JC, Bianchi AL (1979) Evolution de la latence d'envahissement antidromique des neurones respiratoires bulbaires. J Physiol Paris 75: 783–803
Berger AJ (1979) Phrenic motoneurones in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol 42: 76–90
Berger AJ, Cameron WE, Averill DB, Kramis RC, Binder MD (1984) Spatial distribution of phrenic and medial gastrocnemius motoneurons in the cat spinal cord. Exp Neurol 86: 559–575
Brock LG, Coombs JS, Eccles JC (1953) Intracellular recording from antidromically activated motoneurones. J Physiol 122: 429–461
Calvin WH, Schwindt PC (1972) Step in production of motoneuron spikes during rhythmic firing. J Neurophysiol 35: 297–310
Cameron WE, Averill DB, Berger AJ (1983) Morphology of cat phrenic motoneurons as revealed by intrecellular injection of horseradish peroxidase. J Comp Neurol 219: 78–80
Coggeshall RE, Coulter JD, Willis WD (1973) Unmyelinated axons in the ventral roots. Brain Res 57: 229–233
Cohen MI (1973) Synchronization of discharge, spontaneous and evoked, between inspiratory neurons. Acta Neurobiol Exp 33: 189–218
Cullheim S, Kellerth JO, Conradi S (1977) Evidence for direct synaptic interconnections between cat spinal alpha motoneurons via recurrent axon collaterals: a morphological study using intracellular injection of horseradish peroxidase. Brain Res 132: 1–10
Cullheim S, Lipsenthal L, Burke RE (1984) Direct monosynaptic contacts between type-identified alpha motoneurones in the cat. Brain Res 308: 196–199
Eccles JC (1955) The central action of antidromic impulses in motor nerve fibres. Pflügers Arch 260: 385–415
Gauthier P, Barillot JC, Dussardier M (1980) Mise en évidence d'interactions d'origine centrale entre motoneurones laryngés. J Physiol Paris 76: 647–661
Gill PK, Kuno M (1963) Properties of phrenic motoneurones. J Physiol 168: 258–273
Gogan P, Gustafsson B, Jankowska E, Tyc-Dumont S (1984) On re-excitation of feline motoneurones: its mechanism and consequences. J Physiol 350: 81–91
Gogan P, Gueritaud JP, Horcholle-Bossavit G, Tyc-Dumont S (1977) Direct excitatory interactions between spinal motoneurones of the cat. J Physiol 272: 755–767
Goshgarian HG, Rafols JA (1984) The ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J Neurocytol 13: 85–109
Hilaire G, Monteau R (1979) Facteurs déterminant l'ordre de recrutement des motoneurones phréniques. J Physiol Paris 75: 765–781
Hilaire G, Gauthier P, Monteau R (1983a) Central respiratory drive and recruitement order of phrenic and inspiratory laryngeal motoneurones. Resp Physiol 51: 341–359
Hilaire G, Khatib M, Monteau R (1983b) Spontaneous respiratory activity of phrenic and intercostal Renshaw cells. Neurosci Lett 43: 97–101
Hilaire G, Khatib M, Monteau R (1984) Relations excitatrices directes entre motoneurones d'une même population. J Physiol Paris 79: 26 A
Hilaire G, Khatib M, Monteau R (1984) Direct excitatory interactions between motoneurons of a given pool: a new linkage mechanism. In: Bianchi AL, Denavit-Saubié M (eds) Neurogenesis of central respiratory rhythm. MTP Press Ltd, Lancaster, pp 206–208
Kirkwood PA, Sears TA, Stagg D (1976) Short term synchronization of intercostal motoneurone activity. J Physiol 263: 357–381
Lipski J, Fyffe REW, Jodkowski J (1985) Recurrent inhibition of cat phrenic motoneurons. J Neurosci: 5: 1545–1555
Lipski J, Fyffe REW, Jodkowski J (1985) Recurrent inhibition in the control of respiratory motoneurons. In: Bianchi AL, Denavit-Saubie M (eds) Neurogenesis of central respiratory rhythm. MTP Press Ltd, Lancaster, pp 198–205
Martenson A (1963) Reflex responses and recurrent discharges evoked by stimulation of laryngeal nerves. Acta Physiol Scand 57: 248–269
Martenson A (1967) Recurrent discharge studied in single units of laryngeal muscles. Acta Physiol Scand 70: 221–228
Nelson PG (1966) Interaction between spinal motoneurones of the cat. J Neurophysiol 29: 275–287
Renshaw B (1941) Influence of discharge of motoneurones upon excitation of neighbouring motoneurones. J Neurophysiol 4: 167–183
Rikard-Bell GC, Bystrzycka EK (1980) Localization of phrenic motor nucleus in the cat and rabbit studied with horseradish peroxidase. Brain Res 194: 479–483
Schiller HM, Stalberg E (1978) F response studied with single fibre EMG in normal subjects and spastic patients. J Neurol Neurosurg Psychiat 41: 45–53
Sears TA, Stagg D (1976) Short term synchronization of intercostal motoneurone activity. J Physiol 263: 357–381
Sterling P, Kuypers HGJM (1967) Anatomical organization of the brachial spinal cord of the cat. II. The motoneuron plexus. Brain Res 4: 16–32
Ueki S, Koketsu K, Domino EE (1961) Effects of Mecamylamine on the Golgi recurrent collateral Renshaw cell synapse in the spinal cord. Exp Neurol 3: 141–148
Webber CL, Wurster RD, Chung JM (1979) Cat phrenic nucleus architecture as revealed by horseradish peroxidase mapping. Exp Brain Res 35: 395–406
Wilson VJ, Burgess PR (1962) Disinhibition in the cat spinal cord. J Neurophysiol 25: 392–403
Zieglgänsberger W, Reiter C (1974) A cholinergic mechanism in the spinal cord of cats. Neuropharmacology 13: 519–527
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Khatib, M., Hilaire, G. & Monteau, R. Excitatory interactions between phrenic motoneurons in the cat. Exp Brain Res 62, 273–280 (1986). https://doi.org/10.1007/BF00238846
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00238846