Summary
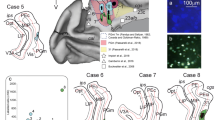
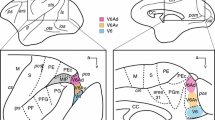
Corticopontine projection patterns were studied after injections of an 3H-leucine and 3H-proline mixture into each of four distinct cortical fields within the inferior parietal lobule and dorsal prelunate gyrus. Different preferential patterns of pontine labeling were observed for each of the four cortical areas studied. Multiple injections across the dorsal aspect of the prelunate gyrus (area DP) yielded scattered patches of label limited to the dorsolateral pontine nuclear region. A single injection within the lateral intraparietal area (area LIP), located in the caudal portion of the lateral bank of the intraparietal sulcus resulted in a series of labeled patches across the dorsal tier of cells stretching across the dorsal portions of the dorsolateral, peduncular and dorsal pontine nuclei. Injection of the cortex on the caudal aspect of the inferior parietal convexity (area 7a) produced multiple patches of label along the lateral margin of the ventral, lateral, and dorsolateral nuclei. Injection of area 7b resulted in label along the lateral aspects of the ventral, lateral and dorsolateral nuclei, as seen with area 7a injections, as well as additional label in the ventromedial portions of the ventral, peduncular and paramedian pontine nuclei. These results provide supporting anatomic evidence for the functional subdivision of the inferior parietal lobule and dorsal aspect of the prelunate gyrus and provide new information about the organization of cortical projections to the primate pontine nuclei.
Similar content being viewed by others
References
Andersen RA, Mountcastle VB (1983) The influence of the angle of gaze upon the excitability of the light-sensitive neurons of the posterior parietal cortex. J Neurosci 3: 532–548
Andersen RA, Siegel RM, Essick GK, Asanuma C (1985a) Subdivision of the inferior parietal lobule and dorsal prelunate gyrus of macaque by connectional and functional criteria. Invest Ophthalmol Vis Sci (Abstr) 26: 266
Andersen RA, Asanuma C, Cowan M (1985b) Callosal and prefrontal associational projecting cell populations in area 7a of the macaque monkey: a study using retrogradely transported fluorescent dyes. J Comp Neurol 232: 443–455
Asanuma C, Andersen RA, Cowan WM (1985) The thalamic relations of the caudal inferior parietal lobule and the lateral prefrontal cortex in monkeys: divergent cortical projections from cell clusters in the medial pulvinar nucleus. J Comp Neurol 241: 357–381
Bailey P, Bonin G von, McCulloch WS (1950) The isocortex of the chimpanzee. University of Illinois Press, Urbana
Baker J, Gibson A, Glickstein M, Stein J (1976) Visual cells in the pontine nuclei of the cat. J Physiol 255: 415–433
Barbus H, Mesulam M-M (1981) Organization of afferent input to subdivisions of area 8 in the rhesus monkey. J Comp Neurol 206: 407–431
Bard P, Woolsey CN, Snider RS (1947) Delimitation of central ervous mechanisms involved in motion sickness. Fed Proc 6: 72
Belnap D, Noda H, Ohmo M (1983) Unit activity and responses to microstimulation in the macaque flocculus during smooth pursuit eye movements. Soc Neurosci Abstr 9: 609
Bonin G von, Bailey P (1947) The neocortex of macaca mulatta. University of Illinois Press, Urbana IL
Brodal P (1978) The corticopontine projection in the rhesus monkey: origin and principles of organization. Brain 101: 251–283
Brodal P (1979) The pontocerebellar projection in the rhesus monkey: an experimental study with retrograde axonal transport of horseradish peroxidase. Neuroscience 4: 193–208
Brodal P (1982a) Further observations on the cerebellar projections from the pontine nuclei and the nucleus reticularis tegmenti pontis in the rhesus monkey. J Comp Neurol 204: 44–55
Brodal P (1982b) The cerebropontocerebellar pathway: salient features of its organization. In: Chan-Palay V, Palay SL (eds) The cerebellum — new vistas. Springer, Berlin, pp 108–132
Brodmann K (1905) Beiträge zur histologischen Localisation der Großhirnrinde. J Psychol Neurol (Leipzig) 4: 177–226
Buchbinder S, Dixon B, Glickstein M, Hwang Y, May J (1980) Visuomotor function in monkeys. Behav Brain Res 2: 248–249
Burne RA, Mihailoff GA, Woodward DJ (1978) Visual corticopontine input to the paraflocculus: a combined autoradiographic and horseradish peroxidase study. Brain Res 143: 139–146
Cohen J, Glickstein M, May J, Robinson F, Stein J (1981) Segregation and overlap of the visual input to the pontine nuclei of macaque. J Physiol 317: 76p
Cohen B, Matsuo U, Raphan T (1977) Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus, J Physiol 270: 321–344
Collewijn H (1972) Latency and gain of the rabbit's optokinetic reactions to small movements. Brain Res 36: 59–70
Cowan WM, Gottlieb DI, Hendrickson AE, Price JL, Woolsey TA (1972) The autoradiographic demonstration of axonal connections in the central nervous system. Brain Res 37: 21–51
Dean P (1976) Effects of inferotemporal lesions on behavior of monkeys. Psychol Bull 83: 41–71
Desimone R, Fleming J, Gross CG (1980) Prestriate afferents to inferior temporal cortex: an HRP study. Brain Res 184: 41–55
Faugier-Grimaud S, Frenois C, Stein D (1978) Effects of posterior parietal lesions on visually guided behavior in monkeys. Neuropsychology 16: 151–168
Fries W (1981) The projection from striate and prestriate visual cortex onto the pontine nuclei in the macaque monkey. Soc Neurosci Abstr 7: 762
Galletti C, Maioli MG, Squatitro S, Battaglini PF (1982) Corticopontine projections from the visual area of the superior temporal sulcus in the macaque monkey. Arch Ital Biol 120: 411–416
Glickstein M, May J (1982) Visual control of movement: the circuits which link visual to motor areas of the brain with special reference to the visual input to the pons and cerebellum. In: Neff WD (ed) Contributions to sensory physiology, Vol VII. Academic Press, New York, pp 103–145
Glickstein M, Cohen J, Dixon B, Gibson A, LaBossiere E, Robinson F (1980) Corticopontine visual projections in macaque monkeys. J Comp Neurol 190: 209–230
Glickstein M, May J, Mercer B (1985) Cortico-pontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol 235: 343–359
Haaxma R, Kuypers GJM (1975) Intrahemispheric cortical connections and visual guidance of hand and finger movements in the rhesus monkey. Brain 98: 239–260
Harting JK (1977) Descending pathways from the superior colliculus: an autoradiographic analysis in the rhesus monkey (Macaca Mulatta). J Comp Neurol 173: 583–612
Hyvärinen J (1981) Regional distribution of functions in parietal association area 7 of the monkey. Brain Res 206: 287–303
Hyvärinen J, Shelepin Y (1979) Distribution of visual and somatic functions in the parietal associative area 7 of the monkey. Brain Res 169: 561–564
Kase M, Noda H, Suzuki DA, Miller DC (1979) Target velocity signals of visual tracking in vermal Purkinje cells of the monkey. Science 205: 717–720
Kase M, Miller DC, Noda H (1980) Discharges of Purkinje ceUs and mossy fibers in the cerebellar vermis of the monkey during saccadic eye movements and fixation. J Physiol (Lond) 300: 539–555
Keller EL, Crandall WF (1983) Neuronal responses to optokinetic stimuli in pontine nuclei of behaving monkey. J Neurophysiol 49: 169–187
Künzle H, Akert K (1977) Efferent connections of cortical area 8 (frontal eye field) in Macaca fascicularis. A reinvestigationusing the autoradiographic technique. J Comp Neurol 173: 147–164
Langer TP, Fuchs AF, Scudder C, Chubb MC (1985) Afferents to the flocculus of the cerebellum in the rhesus macaque as revealed by retrograde transport of horseradish peroxidase. J Comp Neurol 235: 1–25
Leinonen L, Hyvärinen J, Nyman G, Linnankoski I (1979) I. Functional properties of neurons in lateral part of associative area 7 in awake monkeys. Exp Brain Res 34: 299–320
Lisberger S (1982) Role of the cerebellum during motor learning in the vestibulo-ocular reflex. Different mechanisms in different species? Trends Neurosci 5: 437–441
Lisberger SG, Fuchs AF (1978) Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol 41: 733–763
Lynch JC, Graybiel AM (1983) Comparison of afferents traced to the superior colliculus from the frontal eye fields and from two sub-regions of area 7 of the rhesus monkey. Soc Neurosci Abstr 9: 750
Lynch JC, McLaren JW (1982) The contribution of parietooccipital association cortex to the control of slow eye movements. In: Lennerstrand G, Zee D, Keller E (eds) Functional basis of ocular motility disorders. Pergamon Press, New York, pp 501–510
Lynch JC, McLaren JW (1983) Optokinetic nystagmus deficits following parieto-occipital cortex lesions in monkeys. Exp Brain Res 49: 125–130
Lynch JC, Graybiel AM, Lobeck LJ (1985) The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J Comp Neurol 235: 241–254
McElligott JG, Keller EL (1984) Cerebellar involvement in monkey saccadic eye movements: microstimulation. Exp Neurol 86: 543–558
Maguire WM, Baizer JS (1984) Visuotopic organization of the relunate gyrus in rhesus monkey. J Neurosci 4: 1690–1704
Maunsell JHR, Van Essen DC (1983) The connections of the iddle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J Neurosci 3: 2563–2586
May JG, Andersen RA (1984) Different patterns of corticopontine projections from different cortical regions within the inferior parietal lobule and dorsal prelunate gyrus of the monkey. Soc Neurosci Abstr 10: 577
Mishkin M (1972) Cortical visual areas and their interactions. In: Karczmar AG, Eccles JC (eds) Brain and human behavior. Springer, Berlin, pp 187–208
Mustari MJ, Fuchs AJ, Wallman J (1984) Smooth-pursuit-related units in the dorsolateral pons of the rhesus macaque. Soc Neurosci Abstr 10: 987
Newsome WT, Wurtz RH, Dursteler MR, Mikami A (1985) Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci 5: 825–840
Noda H, Suzuki DA (1979a) The role of the flocculus of the monkey in saccadic eye movements. J Physiol (Lond) 294: 317–334
Noda H, Suzuki DA (1979b) The role of the flocculus of the monkey in fixation and smooth pursuit eye movements. J Physiol (Lond) 294: 335–348
Nyby O, Jansen J (1951) An experimental investigation of the cortico-pontine projection in Macaca Mulatta. Skrifter utgitl av det Norske Videnskaps. Akademi: Osla J'Mat Naturv, Klasse H3, pp 1–47
Optican LM, Zee DS, Miles FA, Lisberger SG (1980) Oculomotor deficits in monkeys with flocculus lesions. Soc Neurosci Abstr 6: 474
Ritchie L (1976) Effects of cerebellar lesions on saccadic eye movements. J Neurophysiol 39: 1246–1256
Robinson DA (1976a) The physiology of pursuit eye movements. In: Monty RA and Sender JW (eds) Eye Movements and Psychological Processes. Lawrence Erlbaum Assoc, Publ, New Jersey, pp 19–32
Robinson DA (1976b) Adaptive gain control of vestibulo-ocular reflex by the cerebellum. J Neurophysiol 39: 954–969
Robinson F, Cohen J, May J, Sestokas T, Glickstein M (1984) Cerebellar targets of visual pontine cells in the cat. J Comp Neurol 223: 471–482
Ron S, Robinson DA (1973) Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol 36: 1004–1022
Seltzer B, Pandya DN (1980) Converging visual and somatic sensory cortical input to the intraparietal sulcus of the rhesus monkey. Brain Res 192: 339–351
Shibutani H, Sakata H, Hyvärinen J (1984) Saccade and blinking evoked by microstimulation of the posterior parietal association cortex of the monkey. Exp Brain Res 55: 1–8
Siegal P, Wepsic JG (1974) Alteration of nociception by stimulation of cerebellar structures in the monkey. Physiol Behav 13: 189–194
Sunderland S (1940) The projection of the cerebral cortex on the pons and cerebellum in the macaque monkey. J Anat 74: 201–226
Suzuki DA, Keller EL (1982) Vestibular signals in the posterior vermis of the alert monkey cerebellum. Exp Brain Res 47: 145–147
Suzuki DA, Keller EL (1983) Sensory-oculomotor interactions in primate cerebellar vermis: a role in smooth pursuit control. Soc Neurosci Abstr 9: 606
Suzuki DA, Keller EL (1984) Visual signals in the dorsolateral pontine nucleus of the alert monkey: their relationship to smooth-pursuit eye movements. Exp Brain Res 53: 473–478
Suzuki DA, Noda H, Kase M (1981) Visual and pursuit eye movement-related activity in posterior vermis of monkey cerebellum. J Neurophysiol 46: 1120–1139
Suzuki DA, May J, Keller EL (1984) Smooth-pursuit eye movement deficits with pharmacological lesions in monkey dorsolateral pontine nucleus. Soc Neurosci Abstr 10: 58
Takemori S, Cohen B (1974) Loss of visual suppression of vestibular nystagmus after flocculus lesions. Brain Res 72: 213–224
Ungerleider LG, Mishkin M (1982) Two cortical visual systems. In: Mansfield RJW, Goodale MA (eds) The analysis of visual behavior. MIT Press, Cambridge MA, pp 549–586
Ungerleider LG, Desimone R, Galkin T, Mishkin M (1984) Subcortical projections of area MT in the Macaque. J Comp Neurol 223: 368–386
Van Essen DC, Maunsell JHR (1983) Hierarchical organization and functional streams in the visual cortex. Trends Neurosci 6: 370–375
Waespe W, Henn U (1981) Visual-vestibular interaction in the flocculus of the alert monkey. II. Purkinje cell activity. Exp Brain Res 43: 349–360
Waespe W, Cohen B, Raphan T (1983) Role of the flocculus and para-flocculus in optokinetic nystagmus and visual-vestibular interactions: effects of lesions. Exp Brain Res 50: 9–33
Walker AE (1940) A cytoarchitectural study of the prefrontal area of the macaque monkey. J Comp Neurol 73: 59–86
Wiesendanger R, Wiesendanger M, Ruegg (1979) An anatomical investigation of the corticopontine project in the primate (Macaca Fascicularis and Samiri Sciureus) II. The projection from frontal and parietal association areras. Neuroscience 4: 747–765
Wilson M (1978) Visual system: pulvinar-extrastriate cortex. In: Masterton RB (ed) Handbook of behavioral neurobiology, Vol 1. Plenum Press, New York, pp 209–247
Young LR (1971) Pursuit eye tracking movements. In: Bach-yRita P, Collins CC, Hyde JE (eds) The control of eye movements. Academic Press, New York, pp 429–444
Zee DS, Yamazaki A, Butler, DH, Gucer G (1981) Effects of ablation of flocculus and paraflocculus on eye movements in primate. J Neurophysiol 46: 878–899
Zeki SM (1977) Colour coding in the superior temporal sulcus of rhesus monkey visual cortex. Proc R Soc Lond B 197: 195–223
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
May, J.G., Andersen, R.A. Different patterns of corticopontine projections from separate cortical fields within the inferior parietal lobule and dorsal prelunate gyrus of the macaque. Exp Brain Res 63, 265–278 (1986). https://doi.org/10.1007/BF00236844
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00236844