Summary
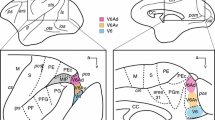
Projections from the posterior thalamic regions to the striatum were studied in the cat by the anterograde tracing method after injecting wheat germ agglutinin-horseradish peroxidase conjugate (WGA-HRP) into the caudalmost regions of the lateroposterior thalamic nucleus (caudal LP), suprageniculate nucleus (Sg) and magnocellular division of the medial geniculate nucleus (MGm). The results were further confirmed by the retrograde tracing method after injecting WGA-HRP into the regions of the caudate nucleus (Cd) and putamen (Put) where afferent fibers from the caudal LP, Sg and MGm were distributed. Fibers from the MGm, Sg or caudal LP were distributed mainly in the medial, middle or lateral part of the caudal half of the putamen (caudal Put), respectively. Although there was a considerable overlap, thalamostriatal fibers from the caudal LP terminated more caudally than those from the MGm. On the other hand, thalamocaudate fibers from the MGm, Sg and lateral part of the caudal LP overlapped with each other in the ventrolateral part of the caudal half of the caudate nucleus (caudal Cd). Fibers from the medial part of the caudal LP were distributed in the ventral part of the caudal Cd. In the superior colliculus (SC) of the cats with WGA-HRP injections in the caudal LP, retrogradely labeled neuronal cell bodies were mainly seen ipsilaterally in the superficial SC layer, and simultaneously, anterogradely labeled axon terminals were observed in the striatum. On the other hand, when WGA-HRP was injected into the Sg or MGm, labeled SC neurons were mainly located in the intermediate and deep SC layers. Thus, ascending impulses from the superficial SC layer may possibly be conveyed ipsilaterally via the caudal LP to the ventral and ventrolateral parts of the caudal Cd and the lateral part of the caudal Put, whereas those from the intermediate and deep SC layers may be relayed via the Sg and/or MGm to the ventrolateral part of the caudal Cd and the middle and medial parts of the caudal Put.
Similar content being viewed by others
Abbreviations
- AC:
-
anterior commissure
- Am:
-
amygdaloid nucleus
- Cd:
-
caudate nucleus
- Ce:
-
centromedial nucleus
- CL:
-
centrolateral nucleus
- Cl:
-
claustrum
- CM-Pf:
-
centre médian-parafascicular complex
- CP:
-
cerebral peduncle
- d:
-
deep SC layer
- EC:
-
external capsule
- Ep:
-
entopeduncular nucleus
- GP:
-
globus pallidus
- i:
-
intermediate SC layer
- IC:
-
internal capsule
- Ip:
-
interpeduncular nucleus
- LG:
-
lateral geniculate nucleus
- LP:
-
lateroposterior nucleus
- MD:
-
mediodorsal nucleus
- MG:
-
medial geniculate nucleus
- MGm:
-
magnocellular division of MG
- MGp:
-
principal division of MG
- NBIC:
-
nucleus of brachium of inferior colliculus
- O:
-
oculomotor nucleus
- OT:
-
optic tract
- Pom:
-
medial division of posterior group of thalamus
- Pt:
-
pretectum
- Pul:
-
pulvinar nucleus
- Put:
-
putamen
- Pv:
-
paraventricular nucleus of thalamus
- R:
-
reticular nucleus of thalamus
- Rh:
-
rhomboid nucleus
- RN:
-
red nucleus
- s:
-
superficial SC layer
- SC:
-
superior colliculus
- Sg:
-
suprageniculate nucleus
- SN:
-
substantia nigra
- SNpc:
-
pars compacta of SN
- SNpr:
-
pars reticulata of SN
- V:
-
lateral ventricle
- VA:
-
ventroanterior nucleus
- VL:
-
ventrolateral nucleus
- VM:
-
ventromedial nucleus
- WGA-HRP:
-
wheat germ agglutinin-HRP conjugate
References
Abrahams VC, Rose PK (1975) Projections of extraocular, neck muscle, and retinal afferents to superior colliculus in the cat: their connections to cells of origin of tectospinal tract. J Neurophysiol 38: 10–18
Abrahams VC, Turner CJ (1981) The nature of afferents from the large dorsal neck muscles that project to the superior colliculus in the cat. J Physiol (Lond) 319: 393–401
Altman J, Carpenter MB (1961) Fiber projections of the superior colliculus in the cat. J Comp Neurol 116: 157–166
Beckstead RM (1984a) The thalamostriatal projection in the cat. J Comp Neurol 223: 313–346
Beckstead RM (1984b) A projection to the striatum from the medial subdivision of the posterior group of the thalamus in the cat. Brain Res 300: 351–356
Berkley KJ (1973) Response properties of cells in ventrobasal and posterior group nuclei of the cat. J Neurophysiol 36: 940–952
Berkley KJ (1980) Spatial relationships between the terminations of somatic sensory and motor pathways in the rostral brainstem of cats and monkeys. I. Ascending somatic sensory inputs to lateral diencephalon. J Comp Neurol 193: 283–317
Berkley KJ, Hand PJ (1978) Efferent projections of the gracile nucleus in the cat. Brain Res 153: 263–283
Bisti SL, Maffei L, Piccolino M (1974) Visuovestibular interactions in the cat superior colliculus. J Neurophysiol 37: 146–155
Bitar M, Saade NE, Banna NR, Jarbur SJ (1981) Interactions of inputs from dorsal columns and ventral tracts with visual inputs on single neurons of cat superior colliculus. Brain Res 220: 356–361
Björkeland M, Boivie J (1984) An anatomical study of the projections from the dorsal column nuclei to the midbrain in cat. Anat Embryol 170: 29–43
Blum PS, Abraham LD, Gilman S (1979) Vestibular, auditory, and somatic input to the posterior thalamus of the cat. Exp Brain Res 34: 1–9
Boivie J (1970) The termination of the cervicothalamic tract in cat. An experimental study with silver impregnation methods. Brain Res 19: 333–360
Boivie J (1971) The termination of the spinothalamic tract in the cat. An experimental study with silver impregnation methods. Exp Brain Res 12: 331–353
Carstens E, Yokota T (1980) Viscerosomatic convergence and responses to intestinal distension of neurons at the junction of midbrain and posterior thalamus in the cat. Exp Neurol 70: 392–402
Chalupa LM, Fish SE (1978) Response characteristics of visual and extravisual neurons in the pulvinar and lateral posterior nuclei of the cat. Exp Neurol 61: 96–120
Droogleever Fortuyn J, Minderhoud JM (1965) Efferent connections of the supra-geniculate nucleus in the albino rat. J Comp Neurol 124: 203–214
Ebner FF (1969) A comparison of primitive forebrain organization in metatherian and eutherian mammals. Ann NY Acad Sci 167: 241–257
Flink R, Wiberg M, Blomqvist A (1983) The termination in the mesencephalon of fibers from the lateral cervical nucleus. An anatomical study in the cat. Brain Res 259: 11–20
Getz B (1952) The termination of spinothalamic fibers in the cat as studied by the method of terminal degeneration. Acta Anat (Basel) 16: 271–290
Glendenning KK, Hall JA, Diamond IT, Hall WC (1975) The pulvinar nucleus of Galago senegalensis. J Comp Neurol 161: 419–458
Godfraind J-M, Meulders M, Veraart C (1972) Visual properties of neurons in pulvinar, nucleus lateralis posterior and nucleus suprageniculatus thalami in the cat. I. Qualitative investigation. Brain Res 44: 503–526
Gordon B (1973) Receptive fields in deep layer of cat superior colliculus. J Neurophysiol 36: 157–178
Graham J (1977) An autoradiographic study of the efferent connections of the superior colliculus in the cat. J Comp Neurol 173: 629–654
Graybiel AM (1970) Some thalamocortical projections of the pulvinar-posterior system of the thalamus in the cat. Brain Res 22: 131–136
Graybiel AM (1972a) Some ascending connections of the pulvinar and nucleus lateralis posterior of the thalamus in the cat. Brain Res 44: 99–125
Graybiel AM (1972b) Some fiber pathways related to the posterior thalamic region in the cat. Brain Behav Evol 6: 363–393
Graybiel AM (1973) The thalamo-cortical projection of the socalled posterior nuclear group: a study with anterograde degeneration methods in the cat. Brain Res 49: 229–244
Hall WC (1972) Visual pathways to the telencephalon in reptiles and mammals. Brain Behav Evol 5: 95–113
Hand PJ, van Winkle T (1976) The efferent connections of the feline nucleus cuneatus. J Comp Neurol 171: 83–110
Harris LR, Blakemore C, Donaghy M (1980) Integration of visual and auditory space in the mammalian superior colliculus Nature (Lond) 288: 56–59
Harting JK, Diamond IT, Hall WC (1973) Anterograde degeneration study of the cortical projections of the lateral geniculate and pulvinar nuclei in the tree shrew (Tupaia glis). J Comp Neurol 150: 393–440
Hazlett JC, Bagley SD (1983) Origin and topography of thalamocaudate projections in the opossum. Neurosci Lett 36: 19–24
Heath CJ (1970) Distribution of axonal degeneration following lesions of the posterior group of thalamic nuclei in the cat. Brain Res 21: 435–438
Heath CJ, Jones EG (1971a) The anatomical organization of the suprasylvian gyrus of the cat. Anat Entwicklungsgesch 45: 7–64
Heath CJ, Jones EG (1971b) An experimental study of ascending connections from the posterior group of thalamic nuclei in the cat. J Comp Neurol 141: 397–426
Hicks TP, Watanabe S, Miyake A, Shoumura K (1984) Organization and properties of visually responsive neurones in the suprageniculate nucleus of the cat. Exp Brain Res 55: 359–367
Higo S, Kawamura S (1984) Topographical linkage of tectothalamo-anterior ectosylvian sulcal cortex in the cat: an 125I-WGA autoradiographic study. Brain Res Bull 12: 647–655
Hikosaka O, Wurtz RH (1983a) Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol 49: 1230–1253
Hikosaka O, Wurtz RH (1983b) Visual and oculomotor functions of monkey substantia nigra pars reticulata. IV. Relation of substantia nigra to superior colliculus. J Neurophysiol 49: 1285–1301
Huerta MF, Harting JK (1984) The mammalian superior colliculus: studies of its morphology and connections. In: Vanegas H (ed) Comparative neurology of the optic tectum. Plenum Press, New York, pp 687–773
Itoh K, Kaneko T, Kudo M, Mizuno N (1984) The intercollicular region in the cat: a possible relay in the parallel somatosensory pathways from the dorsal column nuclei to the posterior complex of the thalamus. Brain Res 308: 166–171
Jones EG, Leavitt RY (1974) Retrograde axonal transport and the demonstration of non-specific projections to the cerebral cortex and striatum from the thalamic intralaminar nuclei in the rat, cat and monkey. J Comp Neurol 154: 349–378
Kalil K (1978) Patch-like termination of thalamic fibers in the putamen of the rhesus monkey: an autoradiographic study. Brain Res 140: 333–339
Kawamura S, Kobayashi E (1975) Identification of laminar origin origin of some tecto-thalamic fibers in the cat. Brain Res 91: 281–285
Kawamura S, Fukushima N, Hattori S, Kudo M (1980) Laminar segregation of cells or origin of ascending projections from the superficial layers of the superior colliculus in the cat. Brain Res 184: 486–490
Killackey HP, Ebner FF (1972) Two different types of thalamocortical projections to a single cortical area in mammals. Brain Behav Evol 6: 141–169
Krauthamer GM (1979) Sensory functions of the neostriatum. In: Divac I, Öberg RGE (ed) The neostriatum. Pergamon Press, Oxford, pp 263–289
Kudo M (1981) Projections of the nuclei of the lateral lemniscus in the cat: an autoradiographic study. Brain Res 221: 57–69
Kudo M, Niimi K (1980) Ascending projections of the inferior colliculus in the cat: an autoradiographic study. J Comp Neurol 191: 545–556
Kudo M, Tashiro T, Higo S, Matsuyama T, Kawamura S (1984) Ascending projections from the nucleus of the brachium of the inferior colliculus in the cat. Exp Brain Res 54: 203–211
Kuljis RO, Fernandez V (1982) On the organization of the retinotecto-thalamo-telencephalic pathways in a chilean rodent: the Octodon degus. Brain Res 234: 189–204
Kunze W, McKenzie JS, Bendrups AP (1979) An electrophysiological study of thalamo-caudate neurons in the cat. Exp Brain Res 36: 233–244
LeVay S, Sherk H (1981) The visual claustrum of the cat. I. Structure and connections. J Neurosci 1: 956–980
Lin C-S, May PJ, Hall WC (1984) Nonintralaminar thalamostriatal projections in the gray squirrel (Sciurus carolinensis) and tree shrew (Tupaia glis). J Comp Neurol 230: 33–46
Mason R (1978) Functional organization in the cat's pulvinar complex. Exp Brain Res 31: 51–66
Mason R (1981) Differential responsiveness of cells in the visual zones of the cat's LP-pulvinar complex to visual stimuli. Exp Brain Res 43: 25–33
Mehler WR (1966a) Further notes on the center median nucleus of Luys. In: Purpura DP, Yahr MD (ed) The thalamus. Columbia University Press, New York, pp 109–122
Mehler WR (1966b) Some observations on secondary ascending afferent systems in the central nervous system. In: Knighton RS, Dumke PR (eds) Pain. Little, Brown and Co, Boston, pp 11–32
Mehler WR (1969) Some neurological species differences — a posteriori. Ann NY Acad Sci 167: 424–468
Mehler WR (1981) The basal ganglia — circa 1982. Appl Neurophysiol 44: 261–290
Mehler WR, Nauta WJH (1974) Connections of the basal ganglia and of the cerebellum. Conf Neurol 36: 205–222
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26: 106–117
Mickle WA, Ades HW (1954) Rostral projection pathway of the vestibular system. Am J Physiol 176: 234–246
Moore RY, Goldberg JM (1963) Ascending projections of the inferior colliculus in the cat. J Comp Neurol 129: 109–136
Nagata K, Kruger L (1979) Tactile neurons of the superior colliculus of the cat: input and physiological properties. Brain Res 174: 19–37
Nauta HJW, Pritz BM, Lasek RJ (1974) Afferents to the rat caudoputamen studied with horseradish peroxidase. An evaluation of a retrograde neuroanatomical research method. Brain Res 67: 219–238
Parent A, Mackey A, De Bellefeuille L (1983) The subcortical afferents to caudate nucleus and putamen in primate: a fluorescence retrograde double labeling study. Neuroscience 10: 1137–1150
Powell EW, Hatton JB (1969) Projections of the inferior colliculus in the cat. J Comp Neurol 136: 183–192
Powell TPS, Cowan WM (1956) A study of thalamostriate relations in the monkey. Brain 79: 364–390
Robson JA, Hall WC (1977) The organization of the pulvinar in the grey squirrel (Sciurus carolinensis). I. Cytoarchitecture and connections. J Comp Neurol 173: 355–388
Royce GJ (1978a) Autoradiographic evidence for a discontinuous projection to the caudate nucleus from the centromedian nucleus in the cat. Brain Res 146: 145–150
Royce GJ (1978b) Cells of origin of subcortical afferents to the caudate nucleus: a horseradish peroxidase study in the cat. Brain Res 153: 465–475
Royce GJ (1983) Single thalamic neurons which project to both the rostral cortex and caudate nucleus studies with the fluorescent double labeling method. Exp Neurol 79: 773–784
Russchen FT (1982) Amygdaloid projections in the cat. II. Subcortical afferent connections. A study with retrograde tracing technique. J Comp Neurol 207: 157–176
Ryugo DK, Killackey HP (1974) Differential telencephalic projections of the medial and ventral divisions of the medial geniculate body of the rat. Brain Res 82: 173–177
Sato M, Itoh K, Mizuno N (1979) Distribution of thalamo-caudate neurons in the cat as demonstrated by horseradish peroxidase. Exp Brain Res 34: 143–153
Stein BE, Arigbede MO (1972) Unimodal and multimodal response properties of neurons in the cat's superior colliculus. Exp Neurol 36: 176–196
Stein BE, Dixon JP (1978) Superior colliculus cells respond to noxious stimuli. Brain Res 158: 65–73
Stein BE, Magalhães-Castro B, Kruger L (1976) Relationship between visual and tactile representations in cat superior colliculus. J Neurophysiol 39: 401–419
Streit P, Reubi JC (1977) A new and sensitive staining method for axonally transported horseradish peroxidase (HRP) in the pigeon visual system. Brain Res 126: 530–537
Sugimoto T, Takada M, Kaneko T, Mizuno N (1984) Substance Ppositive thalamocaudate neurons in the center median-parafascicular complex in the cat. Brain Res 323: 181–184
Suzuki H, Kato H (1969) Neurons with visual properties in the posterior group of the thalamic nuclei. Exp Neurol 23: 353–365
Symonds LLA, Rosenquist AC, Edwards SB, Palmer LA (1981) Projections of the pulvinar-lateral posterior complex to visual cortical areas in the cat. Neuroscience 6: 1995–2020
Takada M, Itoh K, Sugimoto T, Mizuno N (1985) Topographical projections from the thalamus to the putamen in the cat. Neurosci Lett 54: 207–212
Takada M, Itoh K, Yasui Y, Sugimoto T, Mizuno N (1984) Direct projections from the substantia nigra to the posterior thalamic regions in the cat. Brain Res 309: 143–146
van der Kooy D (1979) The organization of the thalamic, nigral and raphe cells projecting to the medial vs lateral caudateputamen in rat. A fluorescent retrograde double labeling study. Brain Res 169: 381–387
Veening JG, Cornelissen FM, Lieven PAJM (1980) The topical organization of the afferents to the caudatoputamen of the rat. A horseradish peroxidase study. Neuroscience 5: 1253–1268
Wiberg M, Blomqvist A (1984) The projection to the mesencephalon from the dorsal column nuclei. An anatomical study in the cat. Brain Res 311: 225–244
Wickelgren BG (1971) Superior colliculus: some receptive field properties of bimodally responsive cells. Science 173: 69–72
Wise LZ, Irvine DRF (1983) Auditory response properties of neurons in deep layers of cat superior colliculus. J Neurophysiol 49: 674–685
Wurtz RH, Albano JE (1980) Visuo-motor function of the primate superior colliculus. Ann Rev Neurosci 3 (1980) 189–226
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takada, M., Itoh, K., Yasui, Y. et al. Topographical projections from the posterior thalamic regions to the striatum in the cat, with reference to possible tecto-thalamo-striatal connections. Exp Brain Res 60, 385–396 (1985). https://doi.org/10.1007/BF00235934
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00235934