Abstract
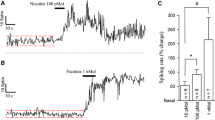
The effects of brainstem microinjections of carbachol on the hippocampal theta rhythm were examined in urethane anesthetized rats. The two most effective theta-eliciting sites with carbachol were the nucleus pontis oralis (RPO) and the acetylcholine-containing pedunculopontine tegmental nucleus (PPT) of the dorsolateral pontine tegmentum. RPO injections generated theta at mean latencies of 38.5±70.8 s and for mean durations of 12.9±5.1 min. Five of seven RPO injections gave rise to theta virtually instantaneously, i.e., before the completion of the injection. PPT injections generated theta at mean latencies of 1.7±1.1 min and for mean durations of 11.9±6.0 min. Injections rostral or caudal to RPO in the caudal midbrain reticular formation (RF) or the caudal pontine RF (nucleus pontis caudalis) generated theta at considerably longer latencies (generally greater than 5 min) or were without effect. Medullary RF injections essentially failed to alter the hippocampal EEG. The finding that theta was produced at very short latencies at RPO suggests that RPO, the putative brainstem source for the generation of theta, is modulated by a cholinergic input. The further demonstration that theta was also very effectively elicited with PPT injections suggests this acetylcholine-containing nucleus of the dorsolateral pons may be a primary source of cholinergic input to RPO in the generation of theta. The hippocampal theta rhythm is a major event of REM sleep. The present results are consistent with earlier work showing that each of the other major events of REM sleep, as well as the REM state, are cholinergically activated at the level of the pontine tegmentum.
Similar content being viewed by others
References
Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA (1984) Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions. Brain Res 306:39–51
Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW, Hobson JA (1987) A neuroanatomical gradient in the pontine tegmentum for the cholinoceptive induction of desynchronized sleep signs. Brain Res 414:245–261
Bland BH (1986) The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol 26:1–54
Bland BH, Whishaw IQ (1976) Generators and topography of hippocampal theta (RSA) in the anesthetized and freely moving rat. Brain Res 118:259–280
Bland BH, Colom LV (1993) Extrinsic and intrinsic properties underlying oscillation and synchrony in limbic cortex. Prog Neurobiol 41:157–208
Colom LV, Ford RD, Bland BH (1987) Hippocampal formation neurons code the level of activation of the cholinergic septohippocampal pathway. Brain Res 410:12–20
Delashaw JB Jr, Foutz A, Guilleminault C, Dement WC (1979) Cholinergic mechanisms and cataplexy in dogs. Exp Neurol 66:745–757
Egan TM, North RA (1986) Aceytlchoine hyperpolarizes central neurones by acting on an M2 muscarinic receptor. Nature 319:405–407
El Mansari M, Sakai K, Jouvet M (1990) Responses of presumed cholinergic mesopontine tegmental neurons to carbachol miroinjections in freely moving cats. Exp Brain Res 83:115–123
George R, Haslett WL, Jenden DJ (1964) A cholinergic mechanism in the brainstem reticular formation: induction of paradoxical sleep. Int J Neuropharmacol 3:541–552
Gnadt JW, Pegram GV (1986) Cholinergic brainstem mechanisms of REM sleep in the rat. Brain Res 384:29–41
Greene RW, Gerber U, McCarley RW (1989) Cholinergic activation of medial pontine reticular formation neurons in vitro. Brain Res 476:154–159
Jones BE (1990) Immunohistochemical study of choline acetyltranferase-immunoreactive processes and cells innervating the pontomedullary reticular formation in the rat. J Comp Neurol 295:485–514
Jones BE (1991) Paradoxical sleep and its chemical/structural substrates in the brain. Neuroscience 40:637–656
Jones BE, Beaudet A (1987) Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study. J Comp Neurol 261:15–32
Katayama Y, DeWitt DS, Becker DP, Hayes RL (1984) Behavioral evidence for a cholinoceptive pontine inhibitory area: descending control of spinal motor output and sensory input. Brain Res 296:241–262
Klemm WR (1972) Effects of electric stimulation of brain stem reticular formation on hippocampal theta rhythm and muscle activity in unaesthetized, cervical and midbrain-transected rats. Brain Res 41:331–334
Kocsis B, Gebber GL, Barman SM, Kenney MJ (1990) Relationships between activity of sympathetic nerve pairs: phase and coherence. Am J Physiol 259:R549-R560
Leonard CS, Llinas R (1988) Electrophysiology of thalamic-projecting cholinergic brainstem neurons and their inhibition by ACH. Neurosci Abstr 14:297
Leonard CS, Llinas R (1990) Electrophysiology of mammalian pedunculopontine and laterodorsal tegmental neurons in vitro: implications for the control of REM sleep. In: Steriade M, Biesold D (eds) Brain cholinergic systems. Oxford University Press, New York, pp 205–223
Macadar AW, Chalupa LM, Lindsley DB (1974) Differentiation of brain stem loci which affect hippocampal and neocortical electrical activity. Exp Neurol 43:499–514
McCormick DA (1990) Cellular mechanisms of cholinegic control of neocortical and thalamic neuronal excitability. In: Steriade M, Biesold D (eds) Brain cholinergic systems. Oxford University Press, New York, pp 236–264
Mitani A, Ito K, Hallanger AE, Wainer BH, Kataoka K, McCarley RW (1988) Cholinergic projections from the laterodorsal and pendunculopontine tegmental nucleus to the pontine gigantocellular tegmental field in the cat. Brain Res 451:397–402
Miller MM, Dement WC (1974) Cataplectic-like behavior in cats after micro-injections of carbachol in pontine reticular formation. Brain Res 68:335–343
Myers RD, Hoch B (1978) 14-C-dopamine microinjected into the brainstem of rat: dispersion kinetics, site content and functional dose. Brain Res Bull 3:601–609
Nunez A, de Andres I, Garcia-Austt E (1991) Relationship of nucleus reticularis pontis caudalis neuronal discharge with sensory and carbachol evoked hippocampal theta rhythm. Exp Brain Res 87:303–308
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, New York
Rye DB, Saper CB, Lee HJ, Wainer BH (1987) Pedunculopontine tegmental nucleus of the rat: cytoarchitecture, cytochemistry, and some extrapyramidal connections of the mesopontine tegmentum. J Comp Neurol 259:483–528
Sakai K, Luppi PH, Salvert D, Kimura H, Maeda Y, Jouvet M (1986) Localization of cholinergic neurons in the cat lower brain stem. C R Acad Sci (Paris) 78:317–324
Sakai K, El Mansari M, Jouvet M (1990) Inhibition by carbachol microinjections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res 527:213–223
Semba K, Reiner PB, Fibiger HC (1990) Single cholinergic mesopontine tegmental neurons project to both the pontine reticular formation and the thalamus in the rat. Neuroscience 38:643–654
Serafin M, Khateb A, Muhlethaler M (1990) Opiates inhibit pedunculopontine neurones in guinea pig brainstem slices. Neurosci Lett 119:125–128
Shiromani PJ, Armstrong DM, Berkowitz A, Jeste DV, Gillin JC (1988a) Distribution of choline acetyltransferase immunoreactive somata in the feline brainstem: implications for REM sleep generation. Sleep 11:1–16
Shiromani PJ, Armstrong DM, Gillin JC (1988b) Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltranseferase immunohistochemical study. Neurosci Lett 95:19–23
Vanderwolf CH (1969) Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol 26:407–418
Van Dongen PAM (1980) Locus ceruleus region: effects on behavior of cholinergic, noradrenergic, and opiate drugs injected intracrebrally into freely moving cats. Exp Neurol 67:52078
Vanni-Mercier G, Sakai K, Lin JS, Jouvet M (1989) Mapping of cholinoceptive brainstem structures responsible for the generation of paradoxical sleep in the cat. Arch Ital Biol 127:133–164
Vertes RP (1977) Selective firing of rat pontine gigantocellular neurons during movement and REM sleep. Brain Res 128:146–152
Vertes RP (1979) Brain stem gigantocellular neurons: patterns of activity during behavior and sleep in the freely moving rat. J Neurophysiol 42:214–228
Vertes RP (1981) An analysis of ascending brain stem systems involved in hippocampal synchronization and desynchronization. J Neurophysiol 46:1140–1159
Vertes RP (1984) Brainstem control of the events of REM sleep. Prog Neurobiol 22:241–288
Vertes RP (1986) Brainstem modulation of the hippocampus: anatomy, physiology and significance. In: Isaacson RL, Pribram KH (eds) The hippocampus, vol 4. Plenum Press, New York, pp 41–75
Vertes RP (1990a) Brainstem mechanisms of slow-wave sleep and REM sleep. In: Klemm WR, Vertes RP (eds) Brainstem mechanisms of behavior, Wiley, New York, pp 535–583
Vertes RP (1990b) Fundamentals of brainstem anatomy: a behavioral perspective. In: Klemm WR, Vertes RP (eds) Brainstem mechanisms of behavior, Wiley, New York, pp 33–103
Webster HH, Jones BE (1988) Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states. Brain Res 458:285–302
Winson J (1974) Patterns of hippocampal theta rhythm in the freely moving rat. Electroencephalogr Clin Neurophysiol 36:291–301
Woolf NJ (1991) Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37:475–524
Yamamoto K, Mamelak AN, Quattrochi JJ, Hobson JA (1990) A cholinoceptive desynchronized sleep induction zone in the anterodorsal pontine tegmentum: spontaneous and drug-induced neuronal activity. Neuroscience 39:295–304
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vertes, R.P., Colom, L.V., Fortin, W.J. et al. Brainstem sites for the carbachol elicitation of the hippocampal theta rhythm in the rat. Exp Brain Res 96, 419–429 (1993). https://doi.org/10.1007/BF00234110
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00234110