Summary
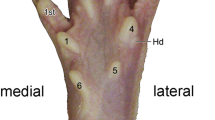
The aim of the present study has been to investigate the spinal projections of cutaneous hindlimb afferents particularly to the deep dorsal horn and to Clarke's column (CC), by using the B-subunit of cholera toxin conjugated to horseradish peroxidase. Injections into three different cutaneous hindlimb nerves in adult rats resulted in dense labeling in the dorsal horn laminae IIi-IV/V, moderate labeling in lamina I and modest labeling in dorsomedial parts of CC. Footpad injections gave similar results, except for a lack of labeling in CC and only weak labeling in laminae I and V. The results suggest that B-HRP should be a useful marker for studying cutaneous myelinated nerve fiber projections to the rat spinal cord.
Similar content being viewed by others
References
Boehme CC (1968) The neural structure of Clarke's nucleus of the spinal cord. J Comp Neurol 132:445–462
Brown AG (1981) Organization in the spinal cord. Springer, Berlin Heidelberg New York
Brown PB, Culberson JL (1981) Somatotopic organization of hindlimb cutaneous dorsal root projections to cat dorsal horn. J Neurophysiol 45:137–143
Brown PB, Fuchs JL (1975) Somatotopic representation of hindlimb skin in cat dorsal horn. J Neurophysiol 38:1–9
Brown AG, Rose PK, Snow PJ (1977) The morphology of hair follicle afferent fibre collaterals in the spinal cord of the cat. J Physiol (Lond) 272:779–797
Brown AG, Rose PK, Snow PJ (1978) Morphology and organization of axon collaterals from afferent fibres of slowly adapting type I units in cat spinal cord. J Physiol (Lond) 277:15–27
Brown AG, Fyffe REW, Noble R (1980) Projections from Pacinian corpuscles and rapidly adapting mechanoreceptors of glabrous skin to the cat's spinal cord. J Physiol (Lond) 307:385–400
Craig AD, Mense S (1983) The distribution of afferent fibers from the gastrocnemius-soleus muscle in the dorsal horn of the cat, as revealed by the transport of horseradish peroxidase. Neurosci Lett 41:233–238
Ganser AL, Kirschner DA, Willinger M (1983) Ganglioside localization on myelinated nerve fibres by cholera toxin binding. J Neurocytol 12:921–938
Hebel R, Stromberg MW (1986) Anatomy and embryology of the laboratory rat. BioMed, Wörthsee
Koerber HR, Brown PB (1982) Somatotopic organization of hindlimb cutaneous nerve projections to cat dorsal horn. J Neurophysiol 48:481–489
Kumazawa T, Pearl ER (1978) Excitation of marginal and substantia gelatinosa neurons in the primate spinal cord: Indications of their place in dorsal horn functional organization. J Comp Neurol 177:417–434
LaMotte C (1977) Distribution of the tract of Lissauer and the dorsal root fibers in the primate spinal cord. J Comp Neurol 172:529–562
Light AR, Durkovic RG (1984) Features of laminar and somatotopic organization of lumbar spinal cord units receiving cutaneous inputs from hindlimb receptive fields. J Neurophysiol 52:449–458
Light AR, Pearl ER (1977) Differential termination of large diameter and small diameter primary afferent fibers in the spinal dorsal gray matter as indicated by labeling with horseradish peroxidase. Neurosci Lett 6:59–63
Light AR, Perl ER (1979a) Reexamination of the dorsal root projection to the spinal dorsal horn including observation on the differential termination of coarse and fine fibers. J Comp Neurol 186:117–132
Light AR, Perl ER (1979b) Spinal termination of functionally identified primary afferent neurons with slowly conducting myelinated fibers. J Comp Neurol 186:133–150
Mense S, Craig AD (1988) Spinal and supraspinal terminations of primary afferent fibers from the gastrocnemius-soleus muscle in the cat. Neurosci 26:1023–1035
Mesulam M-M, Hegarty E, Barbas H, Carson KA, Gower EC, Knapp AG, Moss MB, Mufson EJ (1980) Additional factors influencing sensitivity in the tetrametylbenzidine method for horseradish peroxidase neurohistochemistry. J Histochem Cytochem 28:1255–1259
Meyers DER, Snow PJ (1984) Somatotopically inappropriate projections of single HFA's to cat spinal cord. J Physiol (Lond) 347:59–73
Molander C, Grant G (1985) Cutaneous projections from the rat hindlimb foot to the substantia gelatinosa of the spinal cord studied by transganglionic transport of WGA-HRP conjugate. J Comp Neurol 237:476–484
Molander C, Grant G (1986) Laminar distribution and somatotopic organization of primary afferent fibers from hindlimb nerves in the dorsal horn. A study by transganglionic transport of horseradish peroxidase in the rat. Neuroscience 19:297–312
Molander C, Xu Q, Grant G (1984) The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol 230:133–141
Nyberg G, Blomqvist A (1985) Somatotopic organization of the forelimb cutaneous nerves in brachial dorsal horn: an anatomical study in the cat. J Comp Neurol 242:28–39
Pomeranz B, Wall PD, Weber WV (1968) Cord cells responding to fine myelinated afferents from viscera, muscle and skin. J Physiol (Lond) 199:511–532
Randíc M, Myslinski NR, Gordon JH (1976) Spinal localization of neurons receiving inputs from cutaneous afferents in the cat hindlimb. Brain Res 105:573–577
Réthelyi M (1968) The Golgi architecture of Clarke's column. Acta Morphol Hung 16:311–330
Réthelyi M (1977) Preterminal and terminal axon arborizations in the substantia gelatinosa of cat's spinal cord. J Comp Neurol 172:511–528
Rivero-Melián C, Grant G (1988) Somatotopic organization of hindlimb muscle afferent projections to the column of Clarke of the rat studied with choleragenoid horseradish peroxidase conjugate. Soc Neurosci Abstr 14:1:693
Rivero-Melián C, Grant G (1990a) Lumbar dorsal root projections to spinocerebellar cell groups in the rat spinal cord: a double labeling study. Exp Brain Res 81:85–94
Rivero-Melián C, Grant G (1990b) Distribution of lumbar dorsal root fibers in the lower thoracic and lumbosacral spinal cord of the rat studied with choleragenoid horseradish peroxidase conjugate. J Comp Neurol 299:470–481
Robertson B, Arvidsson J (1985) Transganglionic transport of wheat germ agglutinin-HRP and choleragenoid-HRP in rat trigeminal primary sensory neurons. Brain Res 348:44–51
Robertson B, Grant G (1985) A comparison between wheat germ agglutinin and choleragenoid-horseradish peroxidase as anterogradely transported markers in central branches of primary sensory neurons in the rat with some observations in the cat. Neurosci 14:895–905
Robertson B, Grant G (1989) Immunocytochemical evidence for the localization of the GM1 ganglioside in carbonic anhydrase-containing and RT 97-immunoreactive rat primary sensory neurons. J Neurocytol 18:77–86
Shortland P, Woolf CJ, Fitzgerald M (1989) Morphology and Somatotopic organization of the central terminals of hindlimb hair follicle afferents in the rat lumbar spinal cord. J Comp Neurol 289:416–433
Sugiura Y, Lee CL, Perl ER (1986) Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science 234:358–361
Swett JE, Woolf CJ (1985) The somatotopic organization of primary afferent terminals in the superficial laminae of the dorsal horn of the rat spinal cord. J Comp Neurol 231:66–77
Wall PD (1967) The laminar organization of dorsal horn and effects of descending impulses. J Physiol (Lond) 192:123–144
Woolf CJ (1987) Central terminations of cutaneous mechanoreceptive afferents in the rat lumbar spinal cord. J Comp Neurol 261:105–119
Woolf CJ, Fitzgerald M (1986) Somatotopic organization of cutaneous afferent terminals and dorsal horn neuronal receptive fields in the superficial and deep laminae of the rat lumbar spinal cord. J Comp Neurol 251:517–531
Ygge J, Grant G (1983) The organization of the thoracic spinal nerve projection in the rat dorsal horn demonstrated with transganglionic transport of Horseradish peroxidase. J Comp Neurol 216:1–9
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rivero-Melián, C., Grant, G. Choleragenoid horseradish peroxidase used for studying projections of some hindlimb cutaneous nerves and plantar foot afferents to the dorsal horn and Clarke's column in the rat. Exp Brain Res 84, 125–132 (1991). https://doi.org/10.1007/BF00231767
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00231767