Summary
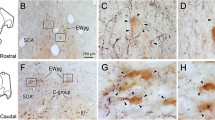
This study describes a regionally selective projection of tyrosine hydroxylase and dopamine β -hydroxylase-immunoreactive fibers from locus coeruleus (LC) and the A4 region of nucleus subcoeruleus to the vestibular nuclear complex in Long-Evans and Sprague-Dawley rats. These fibers travel in two distinct pathways. A lateral descending noradrenergic bundle provides input from LC to the superior vestibular nucleus (SVN), the cochlear nuclei, and the cerebellar cortex. A medial descending noradrenergic bundle provides input to the lateral vestibular nucleus (LVN), medial vestibular nucleus (MVN), and the inferior vestibular nucleus (IVN) before continuing on to the cochlear and cerebellar nuclei. The terminal plexus of these fibers varies markedly across these vestibular nuclear regions. Immunoreactive axons form a dense plexus around somata and proximal dendrites of Deiters' neurons in dorsal LVN. The axon plexus is less dense in SVN and ventral LVN, and relatively sparse in MVN and IVN. This regional selectivity of noradrenergic innervation suggests that central adrenergic systems may selectively modulate vestibulospinal reflexes at the level of the vestibular nuclear complex.
Similar content being viewed by others
References
Aston-Jones G, Foote SL, Bloom FE (1984) Anatomy and physiology of locus coeruleus neurons: functional implications. In: Ziegler MG, Lake CR (eds) Norepinephrine. Williams & Wilkins, Baltimore, pp 92–116
Barnes CD, Manzoni D, Pompeiano O, Stampacchia G, D'Ascanio P (1989) Responses of locus coeruleus and subcoeruleus neurons to sinusoidal neck rotation in decerebrate cat. Neuroscience 31:371–392
Basile AS, Dunwiddie T (1984) Norepinephrine elicits both excitatory and inhibitory responses from Purkinje cells in the in vitro rat cerebellar slice. Brain Res 296:15–25
Billingsley ML, Balaban CD, Berresheim U, Kuhn DM (1986) Comparative studies on the distribution of protein-O-carboxymethyltransferase and tyrosine hydroxylase in rat brain by immunocytochemistry. Neurochem Int 8:255–265
Bloom FE, Algeri S, Groppetti A, Revuelta A, Costa E (1969) Lesions of central norepinephrine terminals with 6-OH-dopamine: biochemistry and fine structure. Science 166:1280–1286
Bloom FE, Hoffer BJ, Siggins GR (1971) Studies on norepinephrine-containing afferents to Purkinje cells of rat cerebellum. I. Localization of the fibers and their synapses. Brain Res 25:501–521
Bloom FE, Krebs H, Nicholson J, Pickel V (1973) The noradrenergic innervation of cerebellar Purkinje cells: localization, function, synaptogenesis, and axonal sprouting of locus coeruleus. In: Fuxe K, Olson L, Zotterman Y (eds) Dynamics of degeneration and growth in neurons. Pergamon Press, Oxford New York, pp 413–423
Cedarbaum JM, Aghajanian GK (1978) Afferent projections to the rat locus coeruleus as determined by a retrograde tracing technique. J Comp Neurol 178:1–16
Charney DS, Woods SW, Price LH, Goodman WK, Glazer WM, Heninger GR (1990) Noradrenergic disregulation in panic disorder. In: Ballenger JC (eds) Neurobiology of panic disorder. Liss, New York, pp 91–105
D'Ascanio P, Pompeiano M, Tononi G (1989a) Inhibition of vestibulospinal reflexes during the episodes of postural atonia induced by unilateral lesion of the locus coeruleus in the decerebrate cat. Arch Ital Biol 127:81–97
D'Ascanio P, Horn E, Pompeiano O, Stampacchia G (1989b) Injections of a beta-adrenergic antagonist in pontine reticular structures modify the gain of vestibulospinal reflexes in decerebrate cats. Arch Ital Biol 127:275–303
Dahlström A, Fuxe K (1964) Evidence for the existence of monamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand 62 [Suppl 232]:1–55
Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63:844–914
Freedman R, Hoffer BJ, Woodward DJ (1975) A quantitative microintophoretic analysis of the responses of central neurons to noradrenaline: interactions with cobalt, manganese, verapamil and dichloroisoprenaline. Brit J Pharmacol 54:529–539
Fritschy J-M, Grzanna R (1989) Immunohistochemical analysis of the neurotoxic effects of DSP-4 identifies two populations of noradrenergic axon terminals. Neuroscience 30:181–197
Fung SJ, Pompeiano O, Barnes CD (1987a) Suppression of the recurrent inhibitory pathway in lumbar cord segments during locus coeruleus stimulation in cats. Brain Res 402:351–354
Fung SJ, Reddy RM, Barnes CD (1987b) Differential labeling of the vestibular complex following unilateral injections of horseradish peroxidase into the cat and rat locus ceoruleus. Brain Res 401:347–352
Fuxe K (1965) Evidence for the existence of monoamine neurons in the central nervous system IV. Distribution of monoamine nerve terminals in the central nervous system. Acta Physiol Scand 62 [Suppl 247]:39–85
Granholm A-CE, Palmer MR (1988) Electrophysiological effects of norepinephrine on Purkinje neurons in intraocular cerebellar grafts α-vs. β -specificity. Brain Res 459:256–264
Hoffer BJ, Siggins GR, Bloom FE (1971) Studies on norepinephrine containing afferents to Purkinje cells of rat cerebellum. II. Sensitivity of Purkinje cells to norepinephrine and related substances administered by microiontophoresis. Brain Res 25:523–534
Hoffer BJ, Siggins GR, Oliver AP, Bloom FE (1973) Activation of the pathway from locus coeruleus to rat cerebellar Purkinje neurons: pharmacologic evidence of noradrenergic central inhibition. J Pharmacol Exp Ther 184:553–569
Ito M (1984) The Cerebellum and Neural Control. Raven Press, New York
Jacob R, Furman JMR, Clark DB, Durrant JD, Balaban CD (1993) Psychogenic dizziness. In: Sharpe JA, Barber HG (eds) The vestibulo-ocular reflex and vertigo. Raven Press, New York
Jacobs BL (1986) Single unit activity of locus coeruleus neurons in behaving animals. Prog Neurobiol 27:183–194
Jones BE, Yang T-Z (1985) The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol 242:56–92
Kirsten EB, Sharma JA (1976) Characteristics and response differences to iontophoretically applied norepinephrine, d-amphetamine and acetylcholine on neurons in the medial and lateral vestibular nuclei of the cat. Brain Res 112:77–90
Kirsten EB, Schoener EP, Wang SC (1974) Effects of d-amphetamine on single vestibular neurons. J Pharmacol Exp Ther 191:377–383
Kössl M, Vater M, Schweizer H (1988) Distribution of catecholamine fibers in the cochlear nucleus of horseshoe bats and moustache bats. J Comp Neurol 269:523–534
Kromer LL, Moore RE (1976) Cochlear nucleus innervation by central norepinephrine neurons in the rat. Brain Res 118:531–537
Kromer LF, Moore RY (1980) Norepinephrine innervation of the cochlear nuclei by locus coeruleus in the bat. Anat Embryol (Berl) 158:227–244
Levitt P, Moore RY (1979) Origin and organization of brainstem catecholamine innervation in the rat. J Comp Neurol 186:505–528
Loizou LA (1969) Projections of the nucleus locus coeruleus in the albino rat. Brain Res 15:563–566
Manzoni D, Pompeiano O, Barnes CD, Stampacchia G, D'Ascanio P (1989) Convergence and interaction of neck and macular vestibular inputs on locus coeruleus and subcoeruleus neurons. Pflugers Arch 413:580–598
McLean, Nakane (1974) Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem 22:1077–1083
Moises HJ, Woodward DJ (1980) Potentiation of GABA inhibitory action in cerebellum by locus coeruleus stimulation. Brain Res 182:327–344
Moises HJ, Woodward DJ, Hoffer BJ, Freedman R (1979) Interactions of norepinephrine cell responses to putative amino acid transmitters applied by microiontophoresis. Exp Neurol 64:493–515.
Moore RY (1982) Catecholamine neuron systems in brain. Ann Neurol 12:321–327
Moore RY, Bloom FE (1979) Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Ann Rev Neurosci 2:113–168
Moore RY, Card JP (1984) Noradrenaline-containing neuron systems. In: Björklund A, Hökfelt T (eds) Classical transmitters in the CNS, part I. Elsevier, Amsterdam New York, pp 123–156
Olsen L, Fuxe K (1971) On the projection from the locus coeruleus noradrenaline neurons: The cerebellar innervation. Brain Res 28:165–171
Palkovits M, Brownstein MJ (1989) Catecholamines in the central nervous system. In: Trendelenburg U, Weiner N (eds) Catecholamines II. Springer, Berlin Heidelberg New York, pp 1–26
Pickel VM, Segal M, Bloom FE (1974) A radiographic study of the efferent pathways of the nucleus locus coeruleus. J Comp Neurol 155:15–42
Pompeiano O (1989) Relationship of noradrenergic locus coeruleus neurones to vestibulo-spinal reflexes. Prog Brain Res 80:329–343
Pompeiano O, Manzoni D, Barnes CD, Stampacchia G, D'Ascanio P (1990) Responses of locus coeruleus and subcoeruleus neurons to sinusoidal stimulation of labyrinthine receptors. Neuroscience 35:227–248
Redmond DE Jr, Huang YH, Snyder D, Maas J (1976) Behavioral effects of stimulation of the nucleus locus coeruleus in the stump-tailed monkey Macaco arctoides. Brain Res 116:502–510
Steinbusch HWM (1991) Distribution of histaminergic neurons and fibers in rat brain. Comparison with noradrenergic and serotonergic innervation of the vestibular system. Acta Otolaryngol (Stockh) Suppl 479:12–23
Swanson LW, Hartman BK (1975) The central adrenergic system. An immunoflourescence study of the location of cell bodies and their efferent connections in the rat utilizing dopamine- β -hydroxylase as a marker. J Comp Neurol 163:467–506
Tanabe M, Ono H, Fukuda H (1990) Spinal alpha 1- and alpha 2-adrenoreceptors mediate facilitation and inhibition of spinal motor transmission, respectively. Jpn J Pharmacol 54:69–77
van der Gugten J, Palkovits M, Wijnen HLJM, Versteg HG (1976) Regional distribution of adrenaline in the rat brain. Brain Res 107:171–175
Versteeg DHG, Van der Gugten J, De Jong W, Palkovits M (1976) Regional concentrations of noradrenaline and dopamine in rat brain. Brain Res 113:563–574
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schuerger, R.J., Balaban, C.D. Immunohistochemical demonstration of regionally selective projections from locus coeruleus to the vestibular nuclei in rats. Exp Brain Res 92, 351–359 (1993). https://doi.org/10.1007/BF00229022
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229022