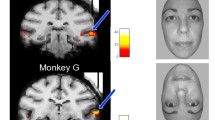
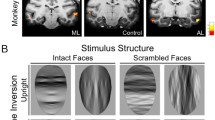
Summary
Neural mechanisms underlying recognition of objects must overcome the changes in an object's appearance caused by inconsistent viewing conditions, particularly those that occur with changes in lighting. In humans, lesions to the posterior visual association cortex can impair the ability to recognize objects and faces across different lighting conditions. Inferotemporal lesions in monkey have been shown to produce a similar difficulty in object matching tasks. Here we report on the extent to which cell responses selective for the face and other views of the head in monkey temporal cortex tolerate changes in lighting. For each cell studied the (preferred) head view eliciting maximal response was first established under normal lighting. Cells were then tested with the preferred head view lit from different directions (i.e. front, above, below or from the side). Responses of some cells failed to show complete generalization across all lighting conditions but together as a “population” they responded equally strongly under all four lighting conditions. Further tests on sub-groups of cells revealed that stimulus selectivity was maintained despite unusual lighting. The cells discriminated between head and control stimuli and between different views of the head independent of the lighting direction. The results indicate that constancy of recognition across different lighting conditions is apparent in the responses of single cells in the temporal cortex. Lighting constancy appears to be established by matching the retinal image to view-specific descriptions of objects (i.e. neurons which compute object structure from a limited range of perspective views).
Similar content being viewed by others
References
Baylis GC, Rolls ET, Leonard CM (1985) Selectivity between faces in the responses of a population of neurons in the cortex of the superior temporal sulcus of the macaque monkey. Brain Res 342:91–102
Benton AL (1980) The neuropsychology of facial recognition. Am Psychol 3:176–186
Benton AL (1990) Facial recognition 1990. Cortex 26:491–499
Benton AL, Van Allen MW (1968) Impairment in facial recognition in patients with cerebral disease. Cortex 4:344–358
Bodamer J (1947) Die Prosop-agnosie. Arch Psychiat Z Neurol 179:6–53
Bruce CJ, Desimone R, Gross CG (1981) Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol 46:369–384
Campbell R, Heywood C, Cowey A, Regard M, Landis T (1990) Sensitivity to eye gaze in macaque monkeys with lesions to the superior temporal cortical ablation and in prosopagnosia. Neuropsychologia 28:1123–1142
Cavanagh P, Leclerc YG (1989) Shape from shadows. J Exp Psychol Human Percept Perform 15:3–27
Cohen M, Grossberg S (1984) Neural dynamics of brightness perception: features, boundaries, diffusion and resonance. Percept Psychophys 36:428–456
Dean P (1976) Effects of inferotemporal lesions on the behaviour of monkeys. Psychol Bull 83:41–71
Desimone R, Albright TD, Gross CG, Bruce C (1984) Stimulusselective properties of inferior temporal neurons in the macaque. J Neurosci 8:2051–2062
Desimone R (1991) Face-selective cells in the temporal cortex of monkeys. J Cogn Neurosci 3:1–8
De Renzi E, Faglioni P, Spinnler H (1968) The performance of patients with unilateral brain damage on face recognition tasks. Cortex 4:17–34
De Renzi E (1986) Current issues on prosopagnosia. In: HD Ellis, NA Jeeves, F Newcombe, A Young (eds) Aspects of face processing. NATO Advanced Science Institute Series. Martinus Nijhoff Publishers, Dordrecht, pp 243–252
Dittrich W (1990) Representation effaces in longtailed macaques. Ethology 85:265–278
Etcoff NL, Freeman R, Cave KR (1991) Can we lose memories of faces? Content specificity and awareness in a prosopagnosic. J Cogn Neurosci 3:25–41
Gershon R, Jepson AD, Tsotsos JK (1986) Ambient illumination and the determination of material changes. J Opt Soc Am 3:1700–1708
Gross CG (1973) Inferotemporal cortex and vision. Prog Physiol Psychol 5:77–123
Harries MH, Perrett DI (1991) Visual processing of faces in temporal cortex: Physiologial evidence for a modular organization and possible anatomical correlates. J Cogn Neurosci 3:9–24
Hasselmo ME, Rolls ET, Baylis GC, Nalwa V (1989) Objectcentred encoding by face-selective neurons in the cortex in the superior temporal sulcus of the monkey. Exp Brain Res 75:417–429
Hecaen H, Angelergues R (1962) Agnosia for faces (prosopagnosia). Arch Neurol 7:92–100
Horn BKP (1975) Obtaining shape from shading information. In: PH Winston (eds) The psychology of computer vision. McGraw-Hill, New York, pp 115–155
Ikeuchi K, Horn BKP (1981) Numerical shape from shading and occluding boundaries. Artif Intell 17:141–184
Johansson G (1973) Visual perception of biological motion and a model for its analysis. Percept Psychophys 14:201–211
Kendrick KM, Baldwin BA (1987) Cells in temporal cortex of conscious sheep can respond preferentially to the sight of faces. Science 236:448–450
Koenderink JJ, van Doom AJ (1980) Photometric invariants related to solid shape. Opt Acta 27:981–996
Lehky SR, Sejnowski TJ (1988) Network model of shape-fromshading: neural function arises from both receptive and projective fields. Nature 333:452–454
Malone DR, Morris HH, Kay MC, Levin HS (1982) Prosopagnosia: a double dissociation between the recognition of familiar and unfamiliar faces. J Neurol Neurosurg Psychiat 45:820–822
Marr D, Nishihara HK (1978) Representation and recognition of the spatial organization of three-dimensional shapes. Proc R Soc Lond B 200:269–294
Meadows JC (1974) The anatomical basis of prosopagnosia. J Neurol Neurosurg Psychiat 37:489–501
Merrill EG, Ainsworth A (1972) Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng 10:662–672
Mishkin M, Ungerleider LG, Macko KA (1983) Object vision and spatial vision: Two cortical pathways. Trends Neurosci 6:414–417
Miyashita Y (1990) Associative representation of visual long term memory in the neurons of the primate temporal cortex In: E Iwai, M Mishkin (eds) Vision, memory and the temporal lobe. Elsevier publishers, New York, pp 75–87
Newcombe F (1969) Missile wounds of the brain: a study of psychological deficits. Oxford University Press, Oxford
Newcombe F, Russell WR (1969) Dissociated visual perceptual and spatial deficits in focal lesions of the right hemisphere. J Neurol Neurosurg Psychiat 32:73–81
Ockleford EM, Milner AD, Dewar W, Sneddon IA (1977) Form perception in stumptail macaques following posterior parietal and lateral frontal lesions. Cortex 13:361–372
Pentland A (1982) Finding the illuminant direction. J Opt Soc Am 72:448–455
Perrett DI, Rolls ET, Caan W (1982) Visual neurons responsive to faces in the monkey temporal cortex. Exp Brain Res 47:329–342
Perrett DI, Smith PAJ, Potter DD, Mistlin AJ, Head AS, Milner AD, Jeeves MA (1984) Neurons responsive to faces in the temporal cortex: studies of functional organization, sensitivity to identity and relation to perception. Hum Neurobiol 3:197–208
Perrett DI, Smith PAJ, Potter DD, Mistlin AJ, Head AS, Milner AD, Jeeves MA (1985) Visual cells in the temporal cortex sensitive to face view and gaze direction. Proc R Soc Lond B 3:293–317
Perrett DI, Mistlin AJ, Chitty AJ, Smith PAJ, Potter DD, Broennimann R, Harries M (1988) Specialized face processing and hemispheric asymmetry in man and monkey: evidence from single unit and reaction time studies. Behav Brain Res 29:245–258
Perrett DI, Harries MH, Bevan R, Thomas S, Benson PJ, Mistlin AJ, Chitty AJ, Hietanen JK, Ortega JE (1989) Frameworks of analysis for the neural representation of animate objects and actions. J Exp Biol 146:87–114
Perrett DI, Harries MH, Benson PJ, Chitty AJ, Mistlin AJ (1990a) Retrieval of structure from rigid and biological motion; an analysis of the visual responses of neurons in the macaque temporal cortex. In: T Troscianko, A Blake (eds) AI and the eye. J Wiley, Chichester, pp 181–201
Perrett DI, Harries MH, Chitty AJ, Mistlin AJ (1990b) Three stages in the classification of body movements by visual neurons. In: HB Barlow, C Blakemore, M Weston-Smith (eds) Images and understanding. Cambridge Univ Press, Cambridge, pp 94–108
Perrett DI, Qram MW, Harries MH, Bevan R, Hietanen JK, Benson PJ, Thomas S (1991a) Viewer-centred and object centred encoding of heads by cells in the superior temporal sulcus of the rhesus monkey. Exp Brain Res 86:159–173
Perrett DI, Benson PJ, Hietanen JK, Oram MW, Dittrich WH (1991b) When is a face not a face? Correlations between perception and single neurones. In R Gregory, J Harris (eds) Anomalies of space, form, colour in perception and art: links with normal and abnormal brain function. (in press)
Rolls ET, Baylis CG (1986) Size and contrast have only small effects on the responses to faces of neurons in the cortex of the superior temporal sulcus of the macaque monkey. Exp Brain Res 65:38–48
Rosene DL, Roy NJ, Davis BJ (1986) A cryoprotection method that facilitates cutting frozen sections of whole monkey brains for histological and histochemical processing without freezing artifact. J Histochem Cytochem 34:1301–1315
Seltzer B, Pandya DN (1978) Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res 149:1–24
Shafer S (1985) Shadows and silhouettes in computer vision. Kluwer Acad Publ, Boston
Snedecor GW, Cochran WG (1980) Statistical methods, 7th edn. Iowa State University Press, Iowa
Todd J, Mingolla E (1983) Perception of surface curvature and direction of illumination from patterns of shading. J Exp Psychol Hum Percept Perform 9:583–595
Tolhurst DJ, Movshon JA, Thompson ID (1981) The dependence of response amplitude and variance of cat visual cortical neurones on stimulus contrast. Exp Brain Res 41:414–419
Ullman S (1979) The interpretation of structure from motion. Proc R Soc Lond B 203:405–426
Warrington EK (1982) Neuropsychological studies of object recognition. Philos Trans R Soc Lond B 298:15–33
Warrington EK, James M (1967) An experimental investigation of facial recognition in patients with unilateral cerebral lesion. Cortex 3:317–326
Weiskrantz L, Saunders RC (1984) Impairments of visual object transforms in monkeys. Brain 107:1033–1072
Whiteley A, Warrington EK (1977) Prosopagnosia: a clinical, psychological and anatomical study of three patients. J Neurol Neurosurg Psychiat 40:395–403
Yamane S, Kaji S, Kawano K (1988) What facial features activate face neurons in the inferotemporal cortex? Exp Brain Res 73:209–214
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hietanen, J.K., Perrett, D.I., Oram, M.W. et al. The effects of lighting conditions on responses of cells selective for face views in the macaque temporal cortex. Exp Brain Res 89, 157–171 (1992). https://doi.org/10.1007/BF00229013
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229013