Summary
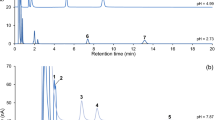
In the present work, differential pulse voltammetry (DPV) measurements of the extracellular fraction of 5-hydroxyindole compounds were performed in rats under long-term chronic conditions. In the nucleus Raphe Dorsalis (n.RD), the voltammetric signal measured at +300 mv (peak 3) disappeared completely 70 to 90 min after injection of Clorgyline (10 mg/kg), a monoamine oxidase inhibitor type A (MAOI-A); the signal measured in such conditions is thus dependent upon extracellular 5-hydroxyindoleacetic acid (5-HIAA peak 3). Deprenyl, an MAOI type B, at the same dose, induced only a slight increase in peak 3 height; according to the fact that MAO-B is selectively located in the 5-HT neurons and since their inhibition does not decrease 5-HIAA peak 3 nor the endogenous 5-HIAA content as measured with High Performance Liquid Chromatography (HPLC), 5-HIAA measured with DPV in the extracellular fluid of untreated animals might come from 5HT released and metabolized by MAO-A outside the 5-HT neurons. In animals implanted for measurements of both voltammetric and polygraphic parameters, the 5-HIAA peak 3 measured mainly in the anterior and ventral part of the n.RD exhibited large increases in its height during slow-wave sleep (SWS: +39%) and paradoxical sleep (PS=+71%) as compared to the waking state (W=100%); these variations could reflect the dendritic release of 5-HT. In the Caudate nucleus (n.Cd) the same voltammetric signal presented reverse fluctuations, i.e. an increase during W and a decrease during SWS and PS. Intracerebroventricular administration of Corticotropin-Like Intermediate lobe Peptide (CLIP, 10 ng/2 μl) induced an increase in PS duration (+51%) preceded and accompanied by an increase in the n.RD 5-HIAA peak 3 height (+50%).
Similar content being viewed by others
References
Arai R, Kimura H, Macola T (1986) Topographic atlas of monoamine oxidase — containing neurons in the rat brain studied by an improved histochemical method. Neuroscience 19:905–925
Borbely AA, Tobler I (1989) Endogenous sleep-promoting substances and sleep regulation. Physiol Reviews 69:605–670
Cespuglio R, Gomez ME, Walker E, Jouvet M (1979) Effets du refroidissement et de la stimulation des noyaux du système du raphe sur les états de vigilance chez le chat. Electroenceph Clin Neurophysiol 47:289–308
Cespuglio R, Faradji H, Gomez ME, Jouvet M (1981a) Single unit recordings in the nuclei raphe dorsalis and magnus during the sleep waking cycle of semi-chronic pretreated cats. Neurosci Lett 24:133–138
Cespuglio R, Faradji H, Ponchon JL, Buda M, Riou F, Gonon F, Pujol JF, Jouvet M (1981b) Differential pulse voltammetry in brain tissue. I. Detection of 5-hydroxyindols in the rat striatum. Brain Res 223:287–298
Cespuglio R, Faradji H, Hahn Z, Jouvet M (1984) Voltammetric detection of brain 5-hydroxyindolamines by means of electrochemically treated carbon fiber electrodes: chronic recording for up to one month with movable cerebral electrodes in the sleeping or waking rat. In: Marsden CA (ed) Measurement of neurotransmitter release in vivo. IBRO handbook series, Vol 6. Wiley and Sons Ltd, Chichester, pp 173–191
Cespuglio R, Sarda N, Gharib H, Faradji H, Jouvet M (1986) Differential pulse voltammetry in vivo with working carbon fiber electrodes: 5-hydroxy-indole compounds or uric acid detection? Exp Brain Res 64:598–595
Cespuglio R, Chastrette N, Jouvet M (1988) Opposite variations of 5-hydroxyindoleacetic (5-HIAA) extracellular concentrations, measured with voltammetry either in the axonal nerve endings or in the cell bodies of the nucleus raphe dorsalis, throughout the sleep-waking cycle. CR Acad Sci (Paris) 307:817–823
Cespuglio R, Chastrette N, Prevautel H, Jouvet M (1989) Serotonin and hypnogenic factors: functional relationship for sleep induction. In: Inoué S and Krueger JP (eds) Endogenous sleep factors. Bouma text. Wassenar, Tokyo, (in press)
Chastrette N, Cespuglio R (1985) Influence of proopiomelanocortin derived peptides on the sleep-waking cycle of the rat. Neurosci Lett 62:365–370
Chastrette N, Cespuglio R, Lin YL, Jouvet M (1989) Proopiomelanocortin (POMC) derived peptides and sleep in the rat. II. Aminergic regulatory processes. Neuropeptides (in press)
Chazal G, Ralston HJ (1987) Serotonin-containing structures in the nucleus raphe dorsalis of the cat: an ultrastructural analysis of dendrites, presynaptic dendrites and axon terminals. J Comp Neurol 259:317–329
Denoyer M, Sallanon M, Kitahama K, Aubert C, Jouvet M (1988) Reversibility of parachlorophenylalanine insomnia by intrahypothalamic microinjection of L 5-hydroxytryptophan. Neurosc 28:83–94
De Simoni MG, Sokola A, Fodritto F, Dal Toso B, Algeri S (1987) Functional meaning of tryptophan-induced increase of 5-HT metabolism as clarified by in vivo voltammetry. Brain Res 411:89–94
Dzoljic MR (1989) Sleep and selective monoamine oxidase B (MAO-B) inhibitors: possible clinical consequences. In: Koella WP (ed) Sleep 88. Gustav Fischer Verlag, Stuttgart New York, pp 219–221
Fornal AC, Jacobs BL (1988) Physiological and behavioral correlates of serotoninergic single-unit activity. In: Osborne NN, Hamon M (eds) Neuronal serotonin. Wiley and Sons Ltd, Chichester, pp 305–345
Fowler CJ, Tipton KF (1982) Deamination of 5-hydroxytryptamine by both forms of monoamine oxidase in the rat brain. J Neurochem 38:733–736
Froment JL, Petitjean F, Bertrand N, Cointy C, Jouvet M (1974) Effets de l'injection intracérébrale de 5–6-Hydroxytryptamine sur les monoamines cérébrales et les états de sommeil du chat. Brain Res 67:405–417
Fueri C, Faudon M, Hery M, Hery F (1984) Release of serotonin from perikarya in cat nodose ganglia. Brain Res 304:173–177
Geffard M, Dulluc J, Rock AM (1985) Antisera against the indolkylamines: tryptophan, 5-hydroxytryptophan, 5-hydroxytryptamine, and 5-methoxytryptamine tested by an enzyme-linked immunosorbent assay method. J Neurochem 44:1221–1228
Gharib A, Reynaud D, Sarda N, Vivien-Roels B, Pevet P, Pacheco H (1989) Adenosine analogs elevate N-acetylserotonin and melatonin in rat pineal gland. Neurosci Lett 106:345–349
Gonon F, Buda M, Cespuglio R, Jouvet M, Pujol JF (1981) Voltammetry in the striatum of chronic freely-moving rats: detection of catechols and ascorbic acid. Brain Res 223:69–80
Jouvet M, Renault J (1966) Insomnie persistante aprés lésions des noyaux du raphe chez le chat. CR Soc Biol (Paris) 160:1461–1465
Jouvet M (1969) Biogenic amines and the states of sleep. Science 163:32–41
Jouvet M (1984) Neuromediateurs et facteurs hypnogènes. Rev Neurol 140:389–400
Jouvet M (1988) The regulation of paradoxical sleep by the hypothalamo-hypophysis. Arch Ital Biol 126:259–274
Johnston JP (1968) Some observations on a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol 17:1285–1297
Kalen P, Strecker RE, Rosengren E, Björklund A (1989) Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res 492:187–202
Kato T, Dong B, Ishii K, Kinemuchi H (1986) Brain dialysis: in vivo metabolism of dopamine and serotonin by monoamine oxidase A but not B in the striatum of unrestained rats. J Neurochem 46:1277–1282
Kitahama K, Kimura H, Maeda T, Jouvet M (1987) Distribution of two types of monoamine oxidase-containing neurons in the cat medulla oblongata demonstrated by an improved histo-chemical method. Neurosci 20:991–999
Knoll J, Magyar K (1972) Some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv Biochem Psychopharmacol 5:393–408
Leger L, Lema F, Chastrette N, Charnay Y, Cespuglio R, Mazie JC, Jouvet M (1990) A monoclonal antibody directed againstCLIP (ACTH 18-39): anatomical distribution of immunoreactivity in the rat brain and hypophysis with quantification of the hypothalamic cell group. J Chem Neuroanat (in press)
Masson-Pevet M, Pevet P (1989) Cytochemical localization of type A and B monoamine oxidase in the rat pineal gland. Cell Tissue Res 255:299–305
McGinty DJ, Harper RM (1976) Dorsal raphe neurons: depression of firing during sleep in cats. Brain Res 101:569–575
Paxinos G, Watson C (1983) The rat brain in stereotaxic coordinates. Academic Press Publishers, New York Paris London, p 85
Petitjean F, Buda C, Janin M, Sallanon M, Jouvet M (1980) L'insomnie provoquée par la p.chlorophenylalanine chez le chat: sa réversibilité par l'injection intraventriculaire d'indolamines. C R Acad Sci 291:1063–1066
Puizillout JJ, Gaudin-Chazal G, Daszuta A, Seyfritz N, Ternaux JP (1979) Release of endogenous serotonin from “encephale isolé” cats. II. Correlations with raphe neuronal activity and sleep and wakefulness. J Physiol (Paris) 75:531–538
Sallanon M, Sakai K, Buda C, Puymartin M, Jouvet M (1986) Augmentation du sommeil paradoxal induite par l'injection d'acide iboténique dans l'hypothalamus ventro latéral postérieur chez le chat. C R Acad Sci (Paris) 303:175–179
Touret M, Kitahama K, Geffard M, Jouvet M (1987) 5-hydroxytryptophan (5-HTP) immunoreactive neurons in the rat brain tissue. Neurosci Lett 80:263–267
Weissmann D, Belin MF, Aguera M, Meunier C, Maitre M, Cash CD, Ehret M, Mandel P, Pujol JF (1987) Immunohistochemistry of tryptophan hydroxylase in the rat brain. Neuroscience 23:291–304
Westlund KN, Denney RM, Kochersperger LM, Rose RM, Abell CW (1985) Distinct monoamine oxidase A and B populations in primate brain. Science 230:181–183
Westlund KN, Denney RM, Rose RM, Abell CW (1988) Localization of distinct monoamine oxidase A and monoamine oxidase B cell populations in human brainstem. Neuroscience 25:439–456
Wolf WA, Youdim MB, Kuhn DM (1985) Does brain 5-HIAA indicate serotonin release or monoamine oxidase activity? Eur J Pharmacol 109:381–387
Zoilla G, Foldi P, Hild G, Szekely AM, Knoll J (1986) The effect of repeated doses of (-) deprenyl on the dynamics of monoaminergic transmission: comparison with clorgyline. Pol J Pharmacol Pharm 38:57–67
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cespuglio, R., Sarda, N., Gharib, A. et al. Voltammetric detection of the release of 5-hydroxyindole compounds throughout the sleep-waking cycle of the rat. Exp Brain Res 80, 121–128 (1990). https://doi.org/10.1007/BF00228853
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00228853