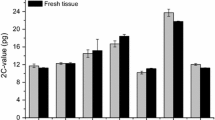
Abstract
A DAPI and ethidium bromide flow cytometric and Feulgen densitometric analysis of genome size variation in Pisum was conducted. The material included 38 accessions of P. sativum of widely different geographic origin and altogether 14 samples of P. elatius, P. abyssinicum, P. humile and P. fulvum. The relative genome size values obtained with the three staining methods were strongly correlated. No evidence for genome size variation was found among P. sativum cultivars. In particular, certain Italian cultivars, for which strongly deviating C-values have been reported, proved to be invariant. The only occasion when ambiguous evidence for marginal genome size variation was found was when all 38 accessions taxonomically affiliated with P. sativum were considered. Pisum abyssinicum and P. fulvum differed from P. sativum by about 1.066-and 1.070-fold, respectively; 1 accession of P. humile differed by 1.089-fold, and 2 of P. elatius by 1.122- and 1.195-fold, respectively (ethidiumbromide comparison), while the other accessions of these taxa were not different from P. sativum. This variation may indicate taxonomic inhomogeneity and demands further investigation. Cultivated P. sativum has long been suspected of not being constant with respect to genome size. As shown here, these findings were not based on genuine differences, but rather were technical in origin.
Similar content being viewed by others
References
Anonymous (1994) Gemeinsamer Sortenkatalog für landwirtschaftliche Pflanzenarten. Pisum sativum L. Amtsbl Eur Gemeinschaft 18:323–346
Arumuganathan K, Earle ED (1991) Nuclear DNA content of some important plant species. Plant Mol Biol Rep 9:208–218
Baranyi M, Greilhuber J (1995) Flow cytometric analysis of genome size variation in cultivated and wild Pisum sativum L. (Fabaceae). Plant Syst Evol 194:231–239
Bennett MD (1985) Intraspecific variation in DNA amount and the nucleotypic dimension in plant genetics. In: Freeling M (ed) Plant Genetics (UCLA symposia on molecular and cellular biology, new series, vol 35). Alan R Liss, New York, pp 283–302
Bennett MD, Smith JB (1976) Nuclear DNA amounts in angiosperms. Philos Trans R Soc London Ser B 274:227–274
Bennett MD, Smith JB (1991) Nuclear DNA amounts in angiosperms. Philos Trans R Soc London Ser B 334:309–345
Bennett MD, Smith JB, Heslop-Harrison JS (1982) Nuclear DNA amounts in angiosperms. Proc R Soc London Ser B 216:179–199
Ben-Ze'ev N, Zohary D (1973) Species relationship in the genus Pisum L. Isr J Bot 22:73–91
Cavallini A, Natali L (1990) Nuclear DNA variability within Pisum sativum (Leguminosae): cytophotometric analyses. Plant Syst Evol 173:179–185
Cavallini A, Cionini G, Gennai D (1993) Nuclear DNA variability within Pisum sativum (Leguminosae): nucleotypic effects on plant growth. Heredity 70:561–565
Conicella C, Errico A (1985) Identification of the chromosomes involved in translocations of P. abyssinicum and P. fulvum. In: Eucarpia Meet Pea Breed. Plant Breeding Center C.N.R., Portici, Italy, pp 86–101
Conicella C, Errico A (1990) Karyotype variations in Pisum sativum ect. abyssinicum. Caryologia 43:87–97
Davis PH (1970) Pisum L. In: Davis PH (ed) Flora of Turkey, vol 3. University Press, Edinburgh, pp 370–372
De Laat AMM, Göhde W, Vogelzang MJDC (1987) Determination of ploidy of single plants and plant populations by flow cytometry. Plant Breed 99:303–307
Doležel J (1991) Flow cytometric analysis of nuclear DNA content in higher plants. Phytochem Anal 2:143–154
Doležel J, Binarova P, Lucretti S (1989) Analysis of nuclear DNA content in plant cells by flow cytometry. Biol Plant 31:113–120
Doležel J, Sgorbati S, Lucretti S (1992) Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol Plant 85:625–631
Errico A, Conicella C, Venora G (1991) Karyotype studies on Pisum fulvum and Pisum sativum, using a chromosome image analysis system. Genome 34:105–108
Galbraith DW, Harkins KR, Maddox JM, Ayres NA, Sharma DP, Firoozabady E (1983) A rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220:1049–1051
Graham MJ, Nickell CD, Rayburn AL (1994) Relationship between genome size and maturity group in soybean. Theor Appl Genet 88:429–432
Greilhuber J (1988) “Self-tanning” — a new and important source of stoichiometric error in cytophotometric determination of nuclear DNA content in plants. Plant Syst Evol 158:87–96
Greilhuber J, Ebert I (1994) Genome size variation in Pisum sativum. Genome 37:646–655
Guerra M dos S (1983) Variaçao no conteúdo de DNA nuclear de Pisum sativum L. Cienc Cult 35:1661–1663
Hâkansson A (1936) Die Reduktionsteilung in einigen Artbastarden von Pisum. Hereditas 21:215–222
Lamprecht H (1964) Partielle Sterilität und Chromosomenstruktur bei Pisum. Agric Hortic Genet 22:56–148
Lamprecht H (1974) Monographie der Gattung Pisum. Steiermärkische Landesdruckerei, Graz.
Lehmann CO (1954) Das morphologische System der Saaterbsen (Pisum sativum L. sens. lat. GOV. ssp. sativum). Zuchter 24:316–337
Lehmann CO, Blixt S (1984) Artificial infraspecific classification in relation to phenotypic manifestation of certain genes in Pisum. Agric Hortic Genet 42:49–74
Le Pecq J-B, Paoletti C (1967) A fluorescent complex between ethidium bromide and nucleic acids. J Mol Biol 27:87–106
Michaelson MJ, Price HJ, Ellison JR, Johnston S (1991) Comparison of plant DNA contents determined by Feulgen microspectrophotometry and laser flow cytometry. Am J Bot 78:183–188
Mukherjee S, Sharma AK (1986) Estimation of in situ DNA content in organs of different strains of Pisum sativum L. Nucleus 28:236–239
Rayburn AL, Auger JA, Benzinger EA, Hepburn AG (1989) Detection of intraspecific DNA content variation in Zea mays L. tby flow cytometry. J Exp Bot 40:1179–1183
Rohlf FJ (1992) BIOM. A package of statistical programs to accompany the text “Biometry”. Applied Biostatistics, New York
Rosen G von (1944) Artkreuzungen in der Gattung Pisum, insbesondere zwischen P. sativum L. und P. abyssinicum Braun. Hereditas 30:261–400
Saccardo F (1971) Crosses among Pisum species. Pisum Newsl 3:38
Schweizer D, Davies D (1972) Nuclear DNA contents of Pisum genotypes grown in vivo and in vitro. Planta 106:23–29
Sgorbati S, Levi M, Sparvoli E, Trezzi F, Lucchini G (1986) Cytometry and flow cytometry of 4',6-diamidino-2-phenylindole (DAPI)-stained suspensions of nuclei released from fresh and fixed tissues of plants. Physiol Plant 68:471–476
Smartt J (1984) Evolution of grain legumes. I. Mediterranean pulses. Exp Agric 20:275–296
Ulrich I, Ulrich W (1986) Flow cytometric DNA-analysis of plant protoplasts stained with DAPI. Z Naturforsch 41c:1052–1056
Ulrich I, Ulrich W (1991) High-resolution flow cytometry of nuclear DNA in higher plants. Protoplasma 165:212–215
Ulrich I, Fritz B, Ulrich W (1988) Application of DNA fluorochromes for flow cytometric DNA analysis of plant protoplasts. Plant Sci 55:151–158
Ulrich W (1992) Simultaneous measurement of DAPI-sulforhodamine 101-stained nuclear DNA and protein in higher plants by flow cytometry. Biotechnic Histochem 67:73–78
Zohary D, Hopf M (1973) Domestication of pulses in the Old World. Science 182:887–894
Author information
Authors and Affiliations
Additional information
Communicated by F. Mechelke
Rights and permissions
About this article
Cite this article
Baranyi, M., Greilhuber, J. Flow cytometric and Feulgen densitometric analysis of genome size variation in Pisum . Theoret. Appl. Genetics 92, 297–307 (1996). https://doi.org/10.1007/BF00223672
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00223672