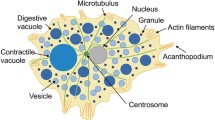
Summary
Fluorescein-labeled muscle actin was microinjected into Amoeba proteus and followed during intracellular redistribution by means of the image-intensification technique. The fully polymerizationcompetent protein becomes part of the endogenous actomyosin system undergoing dynamic changes over time periods of several hours. Singleframe analysis of long-term sequences enabled the direct demonstration of both the contractile activities and morphological transformations of microfilaments in normally locomoting, immobilized and phagocytozing specimens.
In normally locomoting cells the filament layer undergoes continuous changes in spatial distribution depending on the actual pattern of cytoplasmic streaming and cell shape. The highest degree of differentiation is always maintained in the intermediate region between the front and the uroid, thus indicating this segment of the cortex to be the most important site in generating motive force for pseudopodium formation and ameboid movement.
In immobilized cells contracted by the application of ruthenium red or relaxed by different anesthetics, the filament layer forms a continuous thick sheath beneath the cell surface or becomes completely disintegrated. In phagocytozing cells the local polymerization of actin at the tip of pseudopodia forming the food-cup and around the nascent phagosome points to a significant participation of the actomyosin system in the process of capturing and constricting prey organisms.
Although our results provide clear evidence for the overall importance of motive force generation according to the hydraulic pressure theory, some motile phenomena exist in Amoeba proteus that cannot exclusively be explained by this mechanism.
Similar content being viewed by others
References
Allen RD (1968) Differences of a fundamental nature among several types of amoeboid movement. Symp Soc Exp Biol 22:151–168
Allen RD, Allen NS (1978) Cytoplasmic streaming in ameboid movement. Ann Rev Biophys Bioeng 7:469–495
Carrol RC, Butler RG, Morris PA, Gerrard JA (1982) Separable assembly of platelet pseudopodal and contractile cytoskeletons. Cell 30:385–393
Comly LT (1973) Microfilaments in Chaos carolinensis: membrane association, distribution and heavy meromyosin binding in the glycerinated cell. J Cell Biol 58:230–237
Feramisco JR (1979) Microinjection of fluorescently labeled α-actinin into living fibroblasts. Proc Natl Acad Sci USA 76:3967–3971
Gawlitta W, Hinssen H, Stockem W (1980a) The influence of an actin-modulating protein (AM-protein) from Physarum polycephalum on the cell motility of Amoeba proteus. Eur J Cell Biol 23:43–52
Gawlitta W, Stockem W, Wehland J, Weber K (1980b) Organization and spatial arrangement of fluorescein-labelled native actin microinjected into normal locomoting and experimentally influenced Amoeba proteus. Cell Tissue Res 206:181–191
Geiger B (1979) A 130 K protein from chicken gizzard: Its localization at the termini of microfilament bundles in cultured chick cells. Cell 18:193–205
Grebecka L (1980) Reversal of motory polarity of Amoeba proteus by suction. Protoplasma 102:361–375
Grebecka L (1981) Motory effects of perforating peripheral cell layers of Amoeba proteus. Protoplasma 106:343–349
Grebecka L, Grebecki A (1975) Morphometric study of moving Amoeba proteus. Acta Protozool 14:337–361
Grebecka L, Hrebenda B (1979) Topography of cortical layer in Amoeba proteus as related to the dynamic morphology of moving cell. Acta Protozool 18:481–490
Grebecki A (1976) Co-axial motion of the semi-rigid cell frame in Amoeba proteus. Acta Protozool 15:221–248
Grebecki A (1977) Non-axial cell frame movements and the locomotion of Amoeba proteus. Acta Protozool 16:53–85
Grebecki A (1979) Organization of motory functions in amoebae and in slime moulds plasmodia. Acta Protozool 18:43–58
Grebecki A (1980) Behaviour of Amoeba proteus exposed to light-shade difference. Protistologica 16:103–113
Grebecki A (1981) Effects of localized photic stimulation on amoeboid movement and their theoretical implications. Eur J Cell Biol 24:163–175
Grebecki A (1982) Supramolecular aspects of amoeboid movement. Progr in Protozool, Proc VI Internatl Congr Protozool 1:117–130
Grebecki A, Grebecka L (1978) Morphodynamic types of Amoeba proteus: A terminological proposal. Protistologica 14:349–358
Grebecki L, Klopocka W (1981) Functional interdependence of pseudopodia in Amoeba proteus stimulated by light-shade difference. J Cell Sci 50:245–258
Haberey M (1973) Räumliche Anordnung von Plasmafilamenten bei Thecamoeba sphaeronucleolus. Cytobiologie 8:61–75
Haberey M, Stockem W (1971) Amoeba proteus: Morphologie, Zucht und Verhalten. Mikrokosmos 60:33–42
Hauser M (1978) Demonstration of membrane-associated and oriented microfilaments in Amoeba proteus by means of a Schiff base/glutaraldehyde fixative. Cytobiologie 18:95–106
Hoffmann U, Stockem W, Gruber B (in preparation) Dynamics of the cytoskeleton in Amoeba proteus: Influence of different agents on the spatial organization of microinjected IAFlabeled actin
Jeon KW, Jeon MS (1982) Generation of mechanical force during phagocytosis in amoebae. J Cell Biol 95:312a
Keith CH, Feramisco JR, Shelanski M (1981) Direct visualization of fluorescein-labeled microtubules in vitro and in microinjected fibroblasts. J Cell Biol 88:234–240
Klopocka W, Grebecki A (1980) Motory interdependence of pseudopodia in freely moving Amoeba proteus. Acta Protozool 19:129–142
Klopocka W, Grebecki A (1982) Locomotion of Amoeba proteus after standardizing its body shape. Protoplasma 112:37–45
Komnick H, Wohlfahrt-Bottermann KE (1965) Das Grundplasma und die Plasmafilamente der Amoebe Chaos chaos nach enzymatischer Behandlung der Zellmembran. Z Zellforsch 66:434–456
Korn ED (1982) Actin polymerization and its regulation by proteins from non muscle cells. Physiol Rev 62:672–737
Korohoda W, Stockem W (1975) On the nature of hyaline zones in the cytoplasm of Amoeba proteus. Microsc Acta 77:129–141
Kreis TE, Birchmeier W (1980) Stress fiber sarcomeres of fibroblasts are contractile. Cell 22:555–561
Kreis TE, Birchmeier W (1982) Microinjection of fluorescently labeled proteins into living cells with emphasis on cytoskeletal proteins. Int Rev Cytol 75:209–227
Lazarides E, Burridge E (1975) α-Actinin: Immunofluorescent localisation of actin filaments in non-muscle cells. Cell 6:289–298
Mast SO (1932) Localized stimulation, transmission of impulses, and the nature of response in Amoeba. Physiol Zool 5:1–15
Maupin-Szamier P, Pollard TD (1978) Actin filament destruction by osmium tetroxide. J Cell Biol 77:837–852
Nachmias VT (1964) Fibrillar structures in the cytoplasm of Chaos chaos. J Cell Biol 23:183–188
Nachmias VT (1968) Further electron microscope studies on fibrillar organization of the ground cytoplasm of Chaos chaos. J Cell Biol 38:40–52
Pollard TD, Ito S (1970) Cytoplasmic filaments in Amoeba proteus. I. The role of filaments in consistency changes and movement. J Cell Biol 46:267–289
Pollard TD, Korn ED (1973) Electron microscopic identification of actin associated with isolated amoeba plasma membranes. J Biol Chem 248:448–450
Rinaldi RA, Hrebenda B (1975) Oriented thick and thin filaments in Amoeba proteus. J Cell Biol 66:193–198
Sanger JW, Sanger JM, Kreis TE, Jockusch BM (1980) Reversible translocation of cytoplasm actin into the nucleus caused by di-methylsulfoxide. Proc Natl Acad Sci USA 77:5268–5272
Satoh H, Ueda T, Kobatake Y (1982) Primary oscillator of contractional rhythm in the plasmodium of Physarum polycephalum: Role of mitochondria. Cell Struc Func 7:275–283
Schäfer-Danneel S (1967) Strukturelle und funktionelle Voraussetzungen für die Bewegung von Amoeba proteus. Z Zellforsch 78:441–462
Stacey DW, Allfrey VG (1977) Evidence for the autophagy of microinjected proteins in Hela cells. J Cell Biol 75:807–817
Stockem W, Weber K, Wehland J (1978) The influence of microinjected phalloidin on locomotion, protoplasmic streaming and cytoplasmic organization in Amoeba proteus and Physarum polycephalum. Cytobiologie 18:114–131
Stockem W, Hoffmann HU, Gawlitta W (1981) III. Amoeboid movement: Morphological and functional basis of amoeboid movement. Verh Dtsch Zool Ges 71–84
Stockem W, Hoffmann HU, Gawlitta W (1982) Spatial organization and fine structure of the cortical filament layer in normal locomoting Amoeba proteus. Cell Tissue Res 221:505–519
Stockem W, Naib-Majani W, Wohlfahrt-Bottermann KE, Osborn M, Weber K (1983) Pinocytosis and locomotion of amoebae. XIX. Immunocytochemical demonstration of actin and myosin in Amoeba proteus. Eur J Cell Biol 29:171–178
Taylor DL, Condeelis JS (1979) Cytoplasmic structure and contractility in amoeboid cells. Int Rev Cytol 56:57–144
Taylor DL, Wang YL (1978) Molecular cytochemistry: Incorporation of fluorescently labeled actin into living cells. Proc Natl Acad Sci USA 75:857–861
Taylor DL, Wang YL, Heiple JM (1980) Contractile basis of ameboid movement. VII. The distribution of fluorescently labeled actin in living amebas. J Cell Biol 86:590–598
Wang YL, Taylor DL (1979) Distribution of fluorescently labeled actin in living sea urchin eggs during early development. J Cell Biol 82:672–679
Wehland J, Weber K (1980) Distribution of fluorescently labeled actin and tropomyosin after microinjection in living tissue culture cells as observed with TV image intensification. Exp Cell Res 127:397–408
Wehland J, Osborn M, Weber K (1977) Phalloidin-induced actin polymerization in the cytoplasm of cultured cells interferes with cell locomotion and growth. Proc Nat Acad Sci 74:5613–5617
Wehland J, Stockem W, Weber K (1978) Cytoplasmic streaming in Amoeba proteus is inhibited by the actin specific drug phalloidin. Exp Cell Res 115:451–454
Wehland J, Weber K, Gawlitta W, Stockem W (1979) Effects of the actin-binding protein DNAase I on cytoplasmic streaming and ultrastructure of Amoeba proteus. Cell Tissue Res 199:353–373
Author information
Authors and Affiliations
Additional information
The authors wish to thank Dipl. Chem. R. Beck for preparing the IAF-labeled actin and Prof. Dr. D. Kessler for discussion and reading the manuscript. The investigation was supported by a grant (Sto 126/1-3) from the Deutsche Forschungsgemeinschaft.
Rights and permissions
About this article
Cite this article
Stockem, W., Hoffmann, HU. & Gruber, B. Dynamics of the cytoskeleton in Amoeba proteus . Cell Tissue Res. 232, 79–96 (1983). https://doi.org/10.1007/BF00222375
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00222375