Summary
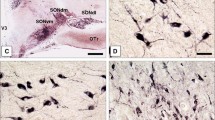
CP-14, a tetradecapeptide from the predicted mutant vasopressin precursor in the homozygous Brattleboro rat was detected immunocytochemically in the supraoptic nucleus of homozygous Brattleboro but not normal rats. The staining was localized to the periphery of the perikarya. CP-14 immunoreactivity was not found in the neural lobes, paraventricular nuclei, accessory nuclei or suprachiasmatic nuclei of either homozygous Brattleboro or normal rats. Vasopressin immunoreactivity was found in the neural lobe and in the perinuclear region of neurons of the supraoptic, paraventricular, suprachiasmatic and accessory nuclei of normal rats. Vasopressin immunoreactivity was also found in homozygous Brattleboro rats, mainly in the ventral part of the supraoptic nucleus: densely stained solitary cells were found amongst other faintly stained perikarya. In both cell-types the staining was mainly in the periphery of the perikarya. No vasopressin immunoreactivity was detected in the paraventricular nuclei, suprachiasmatic nuclei, accessory nuclei or neural lobe of homozygous Brattleboro rats.
CP-14 and vasopressin immunoreactivities were found to be co-localized; both were present in the periphery of the same perikarya of the supraoptic nuclei of homozygous Brattleboro rats. Differential staining was found with antioxytocin serum in both normal rats and homozygous Brattleboro rats: separate neurons were stained for either oxytocin or vasopressin and CP-14. Immunoreactive oxytocin was found mainly in the perinuclear region of the neurons from the supraoptic, paraventricular and accessory nuclei.
Similar content being viewed by others
References
Burbach JPH, De Hoop MJ, Schmale H, Richter D, De Kloet ER, Ten Haaf JA, De Wied D (1984) Differential responses to osmotic stress of vasopressin-neurophysin mRNA in the hypothalamic nuclei. Neuroendocrinology 39:582–584
Dierickx K, Vandesande F (1977) Immunocytochemical localization of the vasopressinergic and oxytocinergic neurons in the human hypothalamus. Cell Tissue Res 184:15–27
Dierickx K, Vandesande F (1979) Immunocytochemical demonstration of separate vasopressin-neurophysin and oxytocin-neurophysin neurons in the human hypothalamus. Cell Tissue Res 196:203–212
Land H, Schütz G, Schmale H, Richter D (1982) Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature 295:299–303
Majzoub JA, Pappey A, Burg R, Habener JF (1984) Vasopressin gene is expressed at low levels in the hypothalamus of the Brattleboro rat. Proc Natl Acad Sci USA 81:5296–5299
Nussey SS, Ang VTY, Jenkins JS, Chowdrey HS, Bisset GW (1984) Brattleboro adrenal contains vasopressin. Nature 310:64–66
Pickering BT, North WG (1982) Biochemical and functional aspects of magnocellular neurons and hypothalamic diabetes insipidus. Ann NY Acad Sci 394:72–81
Pickering BT, Jones CW, Burford GD, McPherson M, Swann RW, Heap PF, Morris JF (1975) The role of neurophysin proteins: suggestions from the study of their transport and turnover. Ann NY Acad Sci 248:15–35
Richards S-J, Morris RJ, Raisman G (1985) Solitary magnocellular neurons in the homozygous Brattleboro rat have vasopressin and glycopeptide immunoreactivity. Neuroscience 16:617–623
Russell JT, Brownstein MJ, Gainer H (1980) Biosynthesis of vasopressin, oxytocin and neurophysins: isolation and characterization of two common precursors (propressophysin and prooxyphysin). Endocrinology 107:1880–1891
Schmale H, Richter D (1984) Single base deletion in the vasopressin gene is the cause of diabetes insipidus in Brattleboro rats. Nature 308:705–709
Schmale H, Heinsohn S, Richter D (1983) Structural organization of the rat gene for the arginine vasopressin-neurophysin precursor. EMBO J 2:763–767
Schmale H, Ivell R, Breindl M, Darmer D, Richter D (1984) The mutant vasopressin gene from diabetes insipidus (Brattleboro) rats is transcribed but the message is not efficiently translated. EMBO J 3:3289–3293
Sokol HW, Zimmerman EA, Sawyer WH, Robinson AG (1976) The hypothalamic-neurohypophysial system of the rat: localization and quantitation of neurophysin by light microscopic immunocytochemistry in normal rats and in Brattleboro rats deficient in vasopressin and a neurophysin. Endocrinology 98:1176–1188
Sternberger L (1979) Immunocytochemistry, 2nd ed. John Wiley and Sons, New York, Chichester, Brisbane, Toronto
Sunde DA, Sokol HW (1975) Quantification of rat neurophysins by polyacrylamide gel electrophoresis (PAGE): Application to the rat with heriditary hypothalamic diabetes insipidus. Ann NY Acad Sci 148:354–364
Swaab DF, Pool CW (1975) Specificity of oxytocin and vasopressin immunofluorescence. J Endocrinol 66:263–272
Swaab DF, Pool CW, Van Leeuwen FW (1977) Can specificity ever be proved in immunocytochemical staining? J Histochem Cytochem 25:388–390
Swann RW, Gonzalez CB, Birkett SD, Pickering BT (1982) Precursors in the biosynthesis of vasopressin and oxytocin in the rat. Characteristics of all the components in high-performance liquid chromatography. Biochem J 208:339–349
Valtin H (1982) The discovery of the Brattleboro rat, recommended nomenclature and the question of proper controls. Ann NY Acad Sci 394:1–9
Valtin H, Sawyer WH, Sokol HW (1965) Neurohypophysial principles in rats homozygous and heterozygous for hypothalamic diabetes insipidus (Brattleboro strain). Endocrinology 77:701–706
Vandesande F, Dierickx K (1975) Identification of the vasopressin producing and of the oxytocin producing neurons in the hypothalamic magnocellular neurosecretory system of the rat. Cell Tissue Res 164:153–162
Vandesande F, Dierickx K (1976) Immunocytochemical demonstration of the inability of the homozygous Brattleboro rat to synthesize vasopressin and vasopressin-associated neurophysin. Cell Tissue Res 165:307–316
Vandesande F, Dierickx K, de Mey J (1975) Identification of the vasopressin-neurophysin II and the oxytocin-neurophysin I producing neurons in the bovine hypothalamus. Cell Tissue Res 165:189–200
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guldenaar, S.E.F., Nahke, P. & Pickering, B.T. Immunocytochemical evidence for the presence of a mutant vasopressin precursor in the supraoptic nucleus of the homozygous Brattleboro rat. Cell Tissue Res. 244, 431–436 (1986). https://doi.org/10.1007/BF00219218
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00219218