Summary
The caudo-dorsal cells (CDC) in the cerebral ganglia of the pond snail Lymnaea stagnalis synthesize the 36-amino acid ovulation hormone (CDCH). We have used immuno-cytochemistry and in situ hybridization to reveal the localization of neurons and axons containing CDCH-like material.
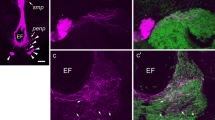
A monoclonal antibody to a fragment of CDCH and a cDNA probe encoding CDCH reacted with the CDC-system, with specific cell groups in the cerebral and pleural ganglia, and with individually occurring neurons throughout the central nervous system. The cells in the pleural ganglia, which were found in about 50% of the preparations studied, are considered as “ectopic” CDC. They are morphologically similar to CDC in their somal dimensions and axonal organization. By means of immuno-electron microscopy it was shown that these neurons contain secretory vesicles that are similar to those of the CDC. The neurons of the bilateral groups occurring in the cerebral ganglia in addition to the CDC are smaller and more intensely stained than the CDC. Axons of these small neurons probably have varicosities located on the CDC axons in the neuropil of the cerebral ganglion, indicating synaptic contacts. Two major axon tracts could be followed from (or toward) the neuropil of the cerebral ganglion. One tract runs from the cerebral gangion via the pleural and parietal ganglia to the visceral ganglion, giving off branches to most nerves emanating from these ganglia. The other tract could be traced through the cerebro-pedal connective to the pedal ganglia. Only in the right pedal ganglion was extensive axonal branching observed. The nerves emanating from this ganglion contained many more immunoreactive axons than those from the left pedal ganglion. A polyclonal antibody raised against the synthetic fragment of CDCH stained, in addition to the neurons and axons revealed with the monoclonal antibody and the cDNA probe, three other major groups of neurons. Two are located in the cerebral ganglion, the other in the left pedal ganglion.
The present findings suggest the presence of a system of neurons that contain CDCH or CDCH-like peptides. The role this system may play in the control of egg-laying and egg-laying behaviour is discussed.
Similar content being viewed by others
References
Chiu AY, Strumwasser F (1981) An immunohistochemical study of the neuropeptidergic bag cells of Aplysia. J Neurosci 1:812–826
Chiu AY, Strumwasser F (1984) Two neuronal populations in the head ganglia of Aplysia californica with egg-laying hormone-like immunoreactivity. Brain Res 294:83–93
Chiu AY, Hunkapillar MW, Heller E, Stuart DK, Hood LE, Strumwasser F (1979) Purification and primary structure of the neuropeptide egg-laying hormone of Aplysia californica. Proc Natl Acad Sci USA 76:6656–6660
Coggeshall RE (1967) A light and electron microscope study of the abdominal ganglion of Aplysia californica. J Neurophysiol 30:1263–1287
Dictus WJAG, Jong-Brink M de, Boer HH (1987) A neuropeptide (calfluxin) is involved in the influx of calcium into mitochondria of the albumen gland of the freshwater snail Lymnaea stagnalis. Gen Comp Endocrinol 65:439–450
Ebberink RHM, Loenhout H van, Geraerts WPM, Joosse J (1985) Purification and amino acid sequence of the ovulation neurohormone of Lymnaea stagnalis. Proc Natl Acad Sci USA 82:7767–7771
Fazekas de St Groth S, Scheidegger D (1980) Production of monoclonal antibodies: strategy and tactics. J Immunol Methods 35:1–21
Frazier WT, Kandel ER, Kupferman I, Waziri R, Coggeshall RE (1967) Morphological and functional properties of identified neurons in the abdominal ganglion of Aplysia californica. J Neurophysiol 30:1288–1351
Geraerts WPM, Bohlken S (1976) The control of ovulation in the hermaphrodite freshwater snail Lymnaea stagnalis by the neurohormone of the caudodorsal cells. Gen Comp Endocrinol 28:350–357
Geraerts WPM, Tensen C, Hogenes ThM (1983) Multiple release of peptides by electrically active neurosecretory caudodorsal cells of Lymnaea stagnalis. Neurosci Lett 41:151–155
Geraerts WPM, Maat A ter, Vreugdenhil E (1987) The peptidergic neuroendocrine control of egg-laying behavior in Aplysia and Lymnaea. In: Laufer H, Downer R (eds) Invertebrate Endocrinology, vol 2. Alan Liss Inc, New York (in press)
Goldschmeding JT, Wilbrink M, Maat A ter (1983) The role of the ovulation hormone in the control of egg-laying behaviour in Lymnaea stagnalis. In: Lever J, Boer HH (eds) Molluscan neuro-endocrinology. North Holland Publishing Company, Amsterdam, pp 251–255
Jansen RF, Bos NPA (1983) Neurophysiology of an identified neuron modulating the ovulation hormone producing caudo-dorsal cells of Lymnaea stagnalis. In: Lever J, Boer HH (eds) Molluscan-neuro-endocrinology. North Holland Publishing Company, Amsterdam, pp 78–81
Jansen RF, Bos NPA (1984) An identified neuron modulating the activity of the ovulation hormone producing caudo-dorsal cells of the pond snail Lymnaea stagnalis. J Neurobiol 15:161–167
Kits KS (1981) Electrical activity and hormone output of ovulation hormone producing neuroendocrine cells in Lymnaea stagnalis (Gastropoda). In: Salanki J (ed) Neurobiology of invertebrates—Mechanisms of integration. Adv Physiol Sci, vol 23. Akadémiai Kiado, Budapest, pp 35–54
Kupferman I (1967) Stimulation of egg laying: possible neuroendocrine functions of bag cells of abdominal ganglion of Aplysia californica. Nature 216:814–815
Mahon AC, Scheller RH (1983) The molecular basis of a neuroendocrine fixed action pattern: egg laying in Aplysia. In: Cold Spring Harbor Symposia on quantitative biology 48:405–412
Mahon AC, Nambu JR, Taussig R, Shyamala M, Roach A, Scheller RH (1985) Structure and expression of the egg-laying hormone gene family in Aplysia. J Neurosci 5:1872–1880
McAllister LB, Scheller RH, Kandel ER, Axel R (1983) In situ hybridization to study the origin and fate of identified neurons. Science 222:800–808
Minnen J van, Vreugdenhil E (1987) The occurrence of gonadotropic hormones in the central nervous system and the reproductive tract of Lymnaea stagnalis. An immunocytochemical and in situ hybridization study. In: Boer HH, Geraerts WPM, Joosse J (eds) Neurobiology. Molluscan models. North Holland Publishing Company, Amsterdam, pp 62–68
Moed PJ, Maat A ter, Bos NPA (1987) The role of release products and second messengers in the regulation of electrical activity of the neuroendocrine caudo-dorsal cells of Lymnaea stagnalis. In: Boer HH, Geraerts WPM, Joosse J (eds) Neurobiology. Molluscan Models. North Holland Publishing Company, Amsterdam, pp 194–200
Painter SD, Kalman VK, Nagle GT, Blankenship JE (1987) Localization of cells containing immunoreactive alpha-bag cell peptide in the central nervous system of Aplysia californica. In: Boer HH, Geraerts WPM, Joosse J (eds) Neurobiology. Molluscan Models. North Holland Publishing Company, Amsterdam, pp 55–62
Rothman BS, Mayeri E, Brown RO, Yuan PM, Shively JE (1983) Primary structure and neuronal effects of α-bag cell peptide, a second candidate neurotransmitter encoded by a single gene in bag cell neurons of Aplysia. Proc Natl Acad Sci USA 80:5753–5757
Rothman BS, Scheller RH, Mayeri E (1985) The bag cell neurons of Aplysia as a possible peptidergic multitransmitter system: From genes to behaviour. In: Zomzely-Neurath C, Walker WA (eds) Gene expression in the brain. John Wiley and Sons Inc, New York, pp 235–274
Roubos EW (1984) Cytobiology of the ovulation-neurohormone producing caudo-dorsal cells of the snail Lymnaea stagnalis. Int Rev Cytol 89:295–346
Roubos EW, Moorer-van Delft CM (1979) Synaptology of the central nervous system of the freshwater snail Lymnaea stagnalis (L), with particular reference to neurosecretion. Cell Tissue Res 198:217–235
Roubos EW, Ven AMH van de, Minnen J van (1987) Immunoelectron microscopy of formation, degradation and exocytosis of ovulation neurohormone in Lymnaea stagnalis. Cell Tissue Res (in press)
Scheller RH, Jackson JF, McAllister LB, Schwartz JH, Kandel ER, Axel R (1982) A family of genes that codes for ELH, a neuropeptide eliciting a stereotyped pattern of behaviour in Aplysia. Cell 28:707–719
Scheller RH, Rothman BS, Mayeri E (1983) A single gene encodes multiple peptide neurotransmitter candidates involved in a stereotyped behaviour. Trends Neurosci 6:340–345
Schmidt ED, Roubos EW (1987) Morphological basis for nonsynaptic communication within the central nervous system by exocytotic release of secretory material from the egg-laying stimulating neuroendocrine caudo-dorsal cells of Lymnaea stagnalis. Neuroscience 20:247–257
Schrier RD, Nelson JA, Oldstone MBA (1985) Detection of human cytomegalovirus in peripheral blood lymphocytes in a natural infection. Science 230:1048–1051
Shyamala M, Nambu JR, Scheller RH (1986) Expression of the egg-laying hormone gene family in the head ganglia of Aplysia. Brain Res 371:49–57
Skowsky WR, Fisher DA (1972) The use of thyroglobulin to induce antigenicity to small molecules. J Lab Clin Med 80:134–144
Vlieger TA de, Kits KS, Maat A ter, Lodder JC (1980) Morphology and electrophysiology of the ovulation hormone producing neuro-endocrine cells of the freshwater snail Lymnaea stagnalis (L). J Exp Biol 84: 259–271
Wendelaar Bonga WE (1970) Ultrastructure and histochemistry of the neurosecretory cells and neurohaemal areas in the pond snail Lymnaea stagnalis (L). Z Zellforsch 108:190–224
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Minnen, J., v.d. Haar, C., Raap, A.K. et al. Localization of ovulation hormone-like neuropeptide in the central nervous system of the snail Lymnaea stagnalis by means of immunocytochemistry and in situ hybridization. Cell Tissue Res. 251, 477–484 (1988). https://doi.org/10.1007/BF00215857
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00215857