Abstract
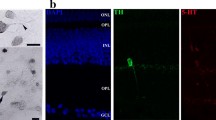
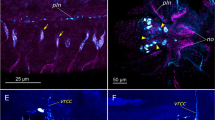
Serotonin-synthesizing neurons in the retina of Xenopus laevis have been identified using anti-phenylalanine hydroxylase (PH) antibody which recognizes tryptophan 5-hydroxylase, the rate-limiting enzyme for serotonin synthesis. Double-labelling experiments, using anti-PH antibody and anti-serotonin antibody/5,7-dihydroxytryptamine (5,7-DHT) uptake, have shown that some serotonin-like immunoreactive/5,7-DHT-labelled neurons exhibit PH-like immunoreactivity (PH-LI) (serotonin-synthesizing neurons), but the others do not (serotonin-accumulating neurons). In the present study, triple-labelling experiments were performed using 5,7-DHT uptake and antibodies raised against GABA and PH, to determine the possible co-localization of γ-aminobutyric acid (GABA) in serotonin-synthesizing and/or -accumulating neurons in the Xenopus retina. All 5,7-DHT-labelled bipolar cells lacked PH-LI; all of them were immunoreactive to GABA. In contrast, all 5,7-DHT-labelled large amacrine cells exhibited PH-LI, but none of them expressed GABA-LI. Small amacrine cells labelled with 5,7-DHT but not PH-LI exhibited GABA-LI, whilst the small amacrine cells with PH-LI lacked GABA-LI. These observations indicate that GABA is co-localized in serotonin-accumulating amacrine and bipolar cells, whereas serotonin-synthesizing large and small amacrine cells do not contain GABA-LI.
Similar content being viewed by others
References
Baker PC, Hoff KM (1971) Melatonin localization in the eyes of larval Xenopus. Comp Biochem Physiol A 39:879–881
Belin M, Nanopoulos D, Didier M, Aguera M, Steinbush H, Verhofstad A, Maitre M, Pujol JF (1983) Immunohistochemical evidence for the presence of gamma-aminobutyric acid and serotonin in one nerve cell. A study on the raphe nuclei of the rat using antibodies to glutamate decarboxylase and serotonin. Brain Res 275:329–339
Bruun A, Ehinger B, Sytsma VM (1984) Neurotransmitter localization in the skate retina. Brian Res 295:233–248
Cahill GM, Besharse JC (1990) Circadian regulation of melatonin in the retina of Xenopus laevis: limitation by serotonin availability. J Neurochem 54:716–719
Ehinger B (1982) Neurotransmitter systems in the retina. Retina 2:305–321
Ehinger B, Floren I (1976) Indoleamine-accumulating neurons in the retina of rabbit, cat and goldfish. Cell Tissue Res 175:37–48
Haan EA, Jennings IG, Cuello AC, Nakata H, Fujisawa H, Chow CW, Kushinsky R, Brittingham J, Cotton RGH (1987) Identification of serotonergic neurons in human brain by a monoclonal antibody binding to all three aromatic amino acid hydroxylases. Brain Res 426:19–27
Gábriel R, Straznicky C, Wye-Dvorak J (1992) GABA-like immunoreactive neurons in the retina of Bufo marinus: evidence for the presence of GABA-containing ganglion cells. Brain Res 571:175–179
Gläsener G, Schmidt C, Himstedt W (1988) Two populations of serotonin-immunoreactive neurons in the frog (Rana esculentd) retina. Neurosci Lett 84:251–254
Ma W, Behar T, Barker JL (1992) Transient expression of GAB A immunoreactivity in the developing rat spinal cord. J Comp Neurol 325:271–290
Marc RE, Liu W-LS, Scholz K, Muller JF (1988) Serotonergic and serotonin-accumulating neurons in the goldfish retina. J Neurosci 8:3427–3450
Massey SC, Mills SL, Marc RE (1992) All indoleamine-accumulating cells in the rabbit retina contain GABA. J Comp Neurol 322:275–291
Millar TJ, Winder C, Ishimoto I, Morgan IG (1988) Putative serotonergic bipolar and amacrine cells in the chicken retina. Brain Res 439:77–87
Millhorn DE, Hökfelt T, Seroogy K, Verhofstad AAJ (1988) Extent of colocalization of serotonin and GABA in neurons of the ventral medulla oblongata in rat. Brain Res 461:169–174
Mitchell CK, Redburn DA (1991) Melatonin inhibits ACh release from rabbit retina. Visual Neurosci 7:479–486
Osborne NN (1982) Evidence for serotonin being a neurotransmitter in the retina. In: Osborne NN (ed) Biology of serotonergic transmission. Wiley, Chichester, pp 401–430
Osborne NN (1988) Retinal serotonin and co-occurrence of serotonin with other neutrotransmitters. In: Osborne NN, Hamon M (eds) Neuronal serotonin. Wiley, New York, pp 129–148
Osborne NN, Beaton DW (1986) Direct histochemical localization of 5,7-dihydroxytryptamine and the uptake of serotonin by a subpopulation of GABA neurons in the rabbit retina. Brain Res 382:158–162
Osborne NN, Nesselhut T, Nicholas DA, Patel S, Cuello C (1982) Serotonin-containing neurons in the vertebrate retinas. J Neurochem 39:1519–1528
Redburn DA (1985) Serotonin neurotransmitter systems in vertebrate retina. In: Morgan RA (ed) Retinal transmitters and modulators: models for the brain, vol 2. Boca Raton, Florida, pp 107–122
Sandell JH, Masland RH (1986) A system of indoleamme-accumulating neurons in the rabbit retina. J Neurosci 6:3331–3347
Schütte M. Witkovsky P (1990) Serotonin-like immunoreactivity in the retina of the clawed frog Xenopus laevis. J Neurocytol 19:504–518
Vaney DI (1986) Morphological identification of serotonin-accumulating neurons in the living retina. Science 233:444–446
Wässle H, Chun MH (1988) Dopaminergic and indoleamine-accumulating amacrine cells express GABA-like immunoreactivity in the cat retina. J Neurosci 8:3383–3394
Wässle H, Voigt T, Patel B (1987) Morphological and immunocytochemical identification of indoleamine-accumulating neurons in the cat retina. J Neurosci 7:1574–1585
Watt CB (1992) A double-label study demonstrating that all serotonin-like immunoreactive amacrine cells in the larval tiger salamander retina express GABA-like immunoreactivity. Brain Res 583:336–339
Weiler R, Schütte M (1985) Morphological and pharmacological analysis of putative serotonergic bipolar and amacrine cells in the retina of a turtle, Pseudemys scripta elegans. Cell Tissue Res 241:373–382
Wiechmann AF, Hollyfield JG (1989) HIOMT-like immunoreactivity in the vertebrate retina: a species comparison. Exp Eye Res 49:1079–1095
Wilhelm M, Zhu B-S, Gábriel R, Straznicky C (1992) Immunocyto-chemical identification of serotonin synthesizing neurons in the vertebrate retina: a comparative study. Exp Eye Res 56:231–240
Yang S-Z, Lam DM-K, Watts CB (1989) Localization of serotonin-like immunoreactive amacrine cells in the larval tiger salamander retina. J Comp Neurol 287:28–37
Yazulla S (1986) GABAergic mechanism in the retina. In: Osborne NN, Chader (eds) Progress in retinal research, vol 5. Pergamon Press, Oxford, pp 51–52
Zhu B-S, Straznicky C (1990) Morphology and distribution of serotonin-like immunoreactive amacrine cells in the retina of Bufo marinus. Visual Neurosci 5:371–378
Zhu B-S, Straznicky C (1992) Large serotonin-like immunoreactive amacrine cells in the retina of developing Xenopus laevis. Dev Brain Res 69:109–116
Zhu B-S, Gábriel R, Straznicky C (1992) Serotonin synthesis and accumulation by neurons of the anuran retina. Visual Neurosci 9:377–388
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Zhu, BS., Straznicky, C. Co-localization of serotonin and GABA in neurons of the Xenopus laevis retina. Anat Embryol 187, 549–555 (1993). https://doi.org/10.1007/BF00214433
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00214433