Abstract
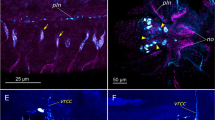
In the sea cucumber, Holothuria scabra, the competent larvae require main settlement organs (SOs), including the ciliary bands (CiBs), tentacles (Ts), podia (PDs), and cues from neurotransmitters, including gamma-aminobutyric acid (GABA) and dopamine (DA), for successful settlement. In the present study, we investigated the spatial distribution of GABA and DA in the developmental stages of H. scabra, with special emphasis on SOs by detecting immunoreactivity (-ir) against these two neurotransmitters. Strong GABA-ir and DA-ir cells and fibers were specifically detected in several SO structures, including CiBs, CiB cells (CiBCs), and long cilia (LCi), of H. scabra larvae. Additionally, we found intense GABA-ir and DA-ir cells in the epithelial lining of bud-papillae (BP) and mesothelium (Me) in the stem (S) region of Ts in larvae and juveniles. Intense GABA-ir and DA-ir were observed in the epineural nerve plexus (ENP) and hyponeural nerve plexus (HNP) of Ts in H. scabra pentactula and juvenile stages. Staining for these two neurotransmitters was particularly intense in the PDs and their nerve fibers. We also found significant changes in the numbers of GABA-ir and DA-ir-positive cells and intensities in the CiBs, Ts, and PDs during the developmental stages. Taken together, we are the first to report on the existence and distribution of GABAergic and dopaminergic systems in structures associated with the settlement. Our findings provide new and important insights into the possible functions of these two neurotransmitters in regulating the settlement of this sea cucumber species.











Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Alfaro AC, Young T, Ganesan AM (2011) Regulatory effects of mussel (Aulacomya maoriana Iredale 1915) larval settlement by neuroactive compounds, amino acids and bacterial biofilms. Aquaculture 322–323:158–168
Amemiya S, Hibino T, Nakano H, Yamaguchi M, Kuraishi R, Kiyomoto M (2015) Development of ciliary bands in larvae of the living isocrinid sea lily Metacrinus rotundus. Acta Zool 96:36–43
Beiras R, Widdows J (1995) Induction of metamorphosis in larvae of the oyster Crassostrea gigas using neuroactive compounds. Mar Biol 123:327–334
Bisgrove BW, Burke RD (1987) Development of the nervous system of the pluteus larva of Strongylocentrotus droebachiensis. Cell Tisssue Res 248:335–343
Bouland C, Massin C, Jangoux M (1982) The fine structure of the buccal tentacles of Holothuria forskali (Echinodermata, Holothuroidea). Zoomorphology 101:133–149
Burke RD (1980) Podial sensory receptors and the induction of metamorphosis in echinoids. J Exp Mar Biol Ecol 47:223–234
Burke RD (1983) Neural control of metamorphosis in Dendraster excentricus. Biol Bull 164:176–188
Cameron JL, Fankboner PV (1984) Tentacle structure and feeding processes in life stages of the commercial sea cucumber Parastichopus californicus (Stimpson). J Exp Mar Biol Ecol 81:193–209
Catapane EJ (1983) Comparative study of the control of lateral ciliary activity in marine bivalves. Comp Biochem Physiol C Comp Pharmacol 75:403–405
Cavey MJ (2006) Organization of the coelomic lining and a juxtaposed nerve plexus in the suckered tube feet of Parastichopus californicus (Echinodermata: Holothuroida). J Morphol 267:41–49
Chaiyamoon A, Tinikul R, Chaichotranunt S, Poomthong T, Suphamungmee W, Sobhon P, Tinikul Y (2018) Distribution and dynamic expression of serotonin and dopamine in the nervous system and ovary of Holothuria scabra during ovarian maturation. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 204:391–407
Chen CP, Tseng CH, Chen BY (1995) The development of the catecholaminergic nervous system in starfish and sea cucumber larvae. Zool Stud 34:248–256
D’Aniello E, Paganos P, Anishchenko E, D’Aniello S, Arnone MI (2020) Comparative neurobiology of biogenic amines in animal models in deuterostomes. Front Ecol Evol 8:587036
Díaz-Balzac CA, Lázaro-Peña MI, Vázquez-Figueroa LD, Díaz-Balzac RJ, García-Arrarás JE (2016) Holothurian nervous system diversity revealed by neuroanatomical analysis. PLoS ONE 11:e0151129
Díaz-Balzac CA, Mejías W, Jiménez LB, García-Arrarás JE (2010) The catecholaminergic nerve plexus of Holothuroidea. Zoomorphology 129:99–109
Dolmatov IY, Ginanova TT, Eliseikina MG, Frolova LT (2018) Formation of the ectodermal organs during the metamorphosis and definitive organogenesis in the holothurian Apostichopus japonicus. Zoomorphology 137:545–564
Dolmatov IY, Ginanova TT, Frolova LT (2016) Metamorphosis and definitive organogenesis in the holothurian Apostichopus japonicus. Zoomorphology 135:173–188
Duy NDQ, Francis DS, Southgate PC (2016) Development of hyaline spheres in late auriculariae of sandfish, Holothuria scabra: is it a reliable indicator of subsequent performance? Aquaculture 465:144–151
Fankboner PV (1978) Suspension-feeding mechanisms of the armoured sea cucumber Psolus chitinoides Clark. J Exp Mar Biol Ecol 31:11–25
Flammang P (1996) Adhesion in echinoderms. In: Studies E (ed) Jangoux M and Lawrence JM). Balkema, Rotterdam, pp 1–60
Flammang P, Conand C (2004) Functional morphology of the tentacles in the apodid holothuroid Synapta maculata. Echinoderms: 327–332
Flammang P, Jangoux M (1993) Functional morphology of coronal and peristomeal podia in Sphaerechinus granularis (Echinodermata, Echinoida). Zoomorphology 113:47–60
Florey E, Cahill MA, Rathmayer M (1975) Excitatory actions of GABA and of acetylcholine in sea urchin tube feet. Comp Biochem Physiol C Toxicol Pharmacol 51:5–12
Gardiner SL, Rieger RM (1980) Rudimentary cilia in muscle cells of annelids and echinoderms. Cell Tissue Res 213:247–252
Hamel J-F, Conand C, Pawson DL, Mercier A (2001) The sea cucumber Holothuria scabra (Holothuroidea: Echinodermata): its biology and exploitation as Beche-de-mer. Adv Mar Biol 41:129–223
Inoue M, Birenheide R, Koizumi O, Kobayakawa Y, Muneoka Y, Motokawa T (1999) Localization of the neuropeptide NGIWYamide in the holothurian nervous system and its effects on muscular contraction. Proc R Soc B 266:993–1000
Kass-Simon G, Pannaccione A, Pierobon P (2003) GABA and glutamate receptors are involved in modulating pacemaker activity in hydra. Comp Biochem Physiol A Mol Integr Physiol 136:329–342
Katow H, Abe K, Katow T, Zamani A, Abe H (2013) Development of the GABA-ergic signaling system and its role in larval swimming in sea urchin. J Exp Biol 216:1704–1716
Katow H, Katow T, Yoshida H, Kiyomoto M, Uemura I (2016) Immunohistochemical and ultrastructural properties of the ciliary band-associated strand (CBAS) in sea urchin Hemicentrotus pulcherrimus larva. Front Zool 13:27
Katow H, Suyemitsu T, Ooka S, Yaguchi J, Jin-nai T, Kuwahara I, Katow T, Yaguchi S, Abe H (2010) Development of a dopaminergic system in sea urchin embryos and larvae. J Exp Biol 213:2808–2819
Katow H, Yoshida H, Kiyomoto M (2020) Initial report of γ-aminobutyric acidergic locomotion regulatory system and its 3-mercaptopropionic acid-sensitivity in metamorphic juvenile of sea urchin, Hemicentrotus pulcherrimus. Sci Rep 10:778
Lacalli T (1993) Ciliary bands in echinoderm larvae: evidence for structural homologies and a common plan. Acta Zool 74:127–133
Lacalli TC, West JE (1986) Ciliary band formation in the doliolaria larva of Florometra: I. The development of normal epithelial pattern. Development 96:303–323
Lacalli TC, West JE (2000) The auricularia-to-doliolaria transformation in two aspidochirote holothurians, Holothuria mexicana and Stichopus californicus. Invertebr Biol 119:421–423
Laverack MS (1974) The structure and function of chemoreceptor cells. In Chemoreception in Marine Organisms: Grant PT and Mackie AM (eds), Academic Press, London, New York, pp 1–48
Marinković M, Berger J, Jékely G (2020) Neuronal coordination of motile cilia in locomotion and feeding. Philos Trans R Soc b: Biol Sci 375:20190165
Martin K, Huggins T, King C, Carroll MA, Catapane EJ (2008) The neurotoxic effects of manganese on the dopaminergic innervation of the gill of the bivalve mollusc, Crassostrea virginica. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 148:152–159
Matsuura H, Yazaki I, Okino T (2009) Induction of larval metamorphosis in the sea cucumber Apostichopus japonicus by neurotransmitters. Fish Sci 75:777–783
McEuen FS, Chia F-S (1991) Development and metamorphosis of two psolid sea cucumbers, Psolus chitonoides and Psolidium bullatum, with a review of reproductive patterns in the family Psolidae (Holothuroidea: Echinodermata). Mar Biol 109:267–279
Mladenov PV, Chia F (1983) Development, settling behaviour, metamorphosis and pentacrinoid feeding and growth of the feather star Florometra serratissima. Mar Biol 73:309–323
Mogami Y, Watanabe K, Ooshima C, Kawano A, Baba SA (1992) Regulation of ciliary movement in sea urchin embryos: dopamine and 5-HT change the swimming behaviour. Comp Biochem Physiol C Comp Pharmacol 101:251–254
Morello SL, Yund PO (2016) Response of competent blue mussel (Mytilus edulis) larvae to positive and negative settlement cues. J Exp Mar Biol Ecol 480:8–16
Morgan A (2008) Metamorphosis in larvae of the temperate sea cucumber Australositchopus mollis. Invertebr Reprod Dev 51:127–143
Morris VB, Selvakumaraswamy P, Whan R, Byrne M (2009) Development of the five primary podia from the coeloms of a sea star larva: homology with the echinoid echinoderms and other deuterostomes. Proc R Soc b: Biol Sci 276:1277–1284
Nakano H, Murabe N, Amemiya S, Nakajima Y (2006) Nervous system development of the sea cucumber Stichopus japonicus. Dev Biol 292:205–212
Nontunha N, Chaiyamoon A, Chaichotranunt S, Tinikul R, Poomtong T, Sobhon P, Tinikul Y (2021) Neurotransmitters induce larval settlement and juvenile growth of the sea cucumber. Holothuria Scabra Aquaculture 535:736427
Nontunha N, Tinikul T, Chaiyamoon A, Vetkama W, Thongbuakaew T, Chaichotranunt S, Poomtong T, Sobhon P, Tinikul Y (2022) The effects of prostaglandin E2 on gonadal development and germ cell proliferation, and its presence during the gonadal cycle in the sea cucumber, Holothuria scabra. Aquaculture 555C:738201
Nontunha N, Tinikul Y, Chaichotranunt S, Poomtong T, Sobhon P, Suphamungmee W (2020) Oocyte development and maturation in the sea cucumber, Holothuria scabra. Agric Nat Resour 54:491–498
Norrevang A, Wingstrand KG (1970) On the occurrence and structure of choanocyte-like cells in some echinoderms. Acta Zool 51:249–270
Pierobon P, Tino A, Minei R, Marino G (2004) Different roles of GABA and glycine in the modulation of chemosensory responses in Hydra vulgaris (Cnidaria, Hydrozoa). Hydrobiologia 530:59–66
Pierrat J, Bédier A, Eeckhaut I, Magalon H, Frouin P (2022) Sophistication in a seemingly simple creature: a review of wild holothurian nutrition in marine ecosystems. Biol Rev Camb Philos Soc 97:273–298
Purcell SW, Hair CA, Mills DJ (2012) Sea cucumber culture, farming and sea ranching in the tropics: progress, problems, and opportunities. Aquaculture 368–369:68–81
Rahman M, Uehara T (2001) Induction of metamorphosis and substratum preference in four sympatric and closely related species of sea urchins (Genus Echinometra) in Okinawa. Zool Stud 40:29–43
Ramofafia C, Byrne M, Battaglene SC (2003) Development of three commercial sea cucumber Holothuria scabra, H. fuscogilva and Actinopyga mauritiana: Larval structure and growth. Mar Freshw Res 54:657–667
Rieger RM, Lombardi J (1987) Ultrastructure of coelomic lining in echinoderm podia: significance for concepts in the evolution of muscle and peritoneal cells. Zoomorphology 107:191–208
Soliman S (1983) Pharmacological control of ciliary activity in the young sea urchin larva. Effects of monoaminergic agents. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 76:181–191
Strathmann RR (1978) The evolution and loss of feeding larval stages of marine invertebrates. Evolution 32:894–906
Sun X, Li Q, Yu H, Kong L (2014) The effect of chemical cues on the settlement of sea cucumber (Apostichopus japonicus) larvae. J Ocean Univ China 13:321–330
Tinikul Y, Kruangkum T, Tinikul R, Sobhon P (2022a) Comparative neuroanatomical distribution and expression levels of neuropeptide F in the central nervous system of the female freshwater prawn, Macrobrachium rosenbergii, during the ovarian cycle. J Comp Neurol 530:729–755
Tinikul Y, Tinikul R, Poljaroen P, Sobhon P (2022b) Differential expression of neuropeptide F during embryogenesis, and its promoting effect on embryonic development of freshwater prawn, Macrobrachium rosenbergii. Aquaculture 555C:738260
Ukena K, Oumi T, Matsushima O, Ikeda T, Fujita T, Minakata H, Nomoto K (1995) Effects of annetocin, an oxytocin-related peptide isolated from the earthworm Eisenia foetida, and some putative neurotransmitters on gut motility of the earthworm. J Exp Zool 272:184–193
Wang H, Bedford FK, Brandon NJ, Moss SJ, Olsen RW (1999) GABA(A)-receptor-associated protein links GABA(A) receptors and the cytoskeleton. Nature 397:69–72
Woodward OM, Willows AO (2006) Nervous control of ciliary beating by Cl–, Ca2+ and calmodulin in Tritonia diomedea. J Exp Biol 209:2765–2773
Zimmer RL (1973) Morphological and developmental affinities of the lophophorates. In: Larwood GP (ed) Living and Fossil Bryozoa. Academic Press, London, pp 593–599
Acknowledgements
We are grateful to Asst. Prof. Dr. Worawit Suphamungmee and Asst. Prof. Dr. Jaruwan Poljaroen, for their useful comments and suggestions. We thank the Center of Nanoimaging (CNI) and the Central Instrument Facility (CIF), Faculty of Science, Mahidol University, for the use of a confocal microscope and instrumental support.
Funding
This research was financially supported by the Agricultural Research Development Agency (public organization). This research project was supported by Mahidol University (Basic Research Fund: fiscal year 2022) (Grant no: BRF1-052/2565) to YT., the Thailand Research Fund (TRF), Thailand Science Research and Innovation, and Mahidol University (TRF Mid-Career Research Grant, RSA6280057) to YT. This work was partially supported by the Graduate Student Thesis Grant of Graduate Studies of Mahidol University Alumni Association, Faculty of Graduate Studies, Mahidol University to NN and YT. This work was financially supported by the Faculty of Science, Mahidol University to YT and RT. This research project was supported by Mahidol University to RT.
Author information
Authors and Affiliations
Contributions
NN and YT: methodology, investigation, validation, formal analysis, writing original draft, and review and editing. SC and TP: resources. YT, PS, and RN: conceptualization, supervision, research design, data analysis, writing review and editing, project administration, and funding acquisition.
Corresponding author
Ethics declarations
Ethics approval
Handling and laboratory protocols involving these sea cucumbers in the present study were carefully performed following the regulations and approval by the Animal Ethics Committee, Faculty of Science, Mahidol University (Animal Protocol number: MUSC62-026–490). All possible effort was made to minimize the number of animals used in this study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nontunha, N., Tinikul, R., Chaichotranunt, S. et al. The presence and distribution of gamma-aminobutyric acid and dopamine during the developmental stages of the sea cucumber, Holothuria scabra, with emphasis on settlement organs. Cell Tissue Res 391, 457–483 (2023). https://doi.org/10.1007/s00441-023-03739-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-023-03739-9