Summary
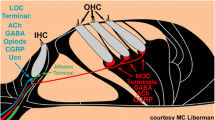
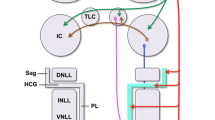
Cholera toxin B subunit conjugated to horseradish peroxidase, and unconjugated cholera toxin B subunit are useful tools for retrograde tract tracing. Unilateral injection of either cholera toxin preparation into the cochlea results in excellent labeling of olivocochlear neurons, as judged by the Golgi-like filling of cell bodies, dendrites, and even axons. By this approach, we have studied the light microscopic cytology and topographic distribution of olivocochlear neurons and counted their numbers in Sprague-Dawley rats. The olivocochlear system of rats can be divided into three subgroups. The lateral olivocochlear system, composed of small cells located exclusively within the ipsilateral lateral superior olive (relative to the test cochlea), and a medial olivocochlear system, composed of large cells bilaterally dispersed within the ventral nucleus of the trapezoid body, conformed to previous topographic descriptions. A third subgroup of approximately 110 large cells, herein termed shell neurons, was labeled by both tracers, but was not well recognized in previous studies. Shell neurons and their dendrites surround the ipsilateral, and to a much lesser extent the contralateral, lateral superior olive. Lateral olivocochlear neurons do not project their dendrites outside the gray matter of the lateral superior olive, while dendrites belonging to shell neurons penetrate into that nucleus as well as into other auditory brain stem nuclei and the surrounding reticular formation. Medial olivocochlear neurons usually project dendrites ventrally into the trapezoid body and are always excluded from the lateral superior olive.
Similar content being viewed by others
Abbreviations
- AChE :
-
acetylcholinesterase
- CGRP :
-
calcitonin generelated peptide
- CT :
-
cholera toxin
- CT-B :
-
cholera toxin B subunit
- CT-HRP :
-
cholera toxin B conjugated horseradish peroxidase
- DAB :
-
3-3′-diamidinobenzidene tetrahydrochloride dihydrate
- DMPO :
-
dorsomedial periolivary region
- DPO :
-
dorsal periolivary region
- GABA :
-
γ-aminobutyric acid
- HRP :
-
horseradish peroxidase
- IHC :
-
inner hair cell
- LOGS :
-
lateral olivocochlear system
- LSO :
-
lateral superior olive
- LVPO :
-
lateral ventral periolivary nucleus
- MDPO :
-
mediodorsal periolivary region
- MNTB :
-
medial nucleus of the trapezoid body
- MOCS :
-
medial olivocochlear system
- MPO :
-
medial periolivary region
- MSO :
-
medial superior olive
- OC :
-
olivocochlear
- OHC :
-
outer hair cell
- pt :
-
pyramidal tract
- SOC :
-
superior olivary complex
- SPN :
-
superior paraolivary nucleus
- tb :
-
trapezoid body
- TMB :
-
3-3′,5-5′ tetramethylbenzidine
- VNTB :
-
ventral nucleus of the trapezoid body
- WGA-HRP :
-
wheat germ agglutinin conjugated horseradish peroxidase
References
Adams JC (1981) Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem 29:775
Adams JC (1989) Non-olivocochlear cholinergic periolivary cells. Soc Neurosci Abst 15:1114
Aschoff A, Ostwald J (1987) Different origins of cochlear efferents in some bat species, rats, and guinea pigs. J Comp Neurol 264:56–72
Aschoff A, Ostwald J (1988) Distribution of cochlear efferents and olivo-collicular neurons in the brain stem of rat and guinea pig. Exp Brain Res 71:241–251
Contreras RJ, Gomez M, Norgren R (1980) Central origins of cranial nerve parasympathetic neurons in the rat. J Comp Neurol 190:373–394
Dallos P, Geisler CD, Matthews JW, Ruggero MA, Steele CR (1990) The mechanisms and biophysics of hearing. Springer, Heidelberg New York, pp 1–418
Ericson H, Blomqvist A (1988) Tracing of neuronal connections with cholera toxin subunit B: light and electron microscopic immunohistochemistry using monoclonal antibodies. J Neurosci Methods 24:225–235
Helfert RH, Schwartz IR, Ryan AF (1988) Ultrastructural characterization of gerbil olivocochlear neurons based on differential uptake of 3-H-D-aspartic acid and wheat germ agglutinin-horseradish peroxidase conjugate from the cochlea. J Neurosci 8:3111–3128
Heyningen van S (1973) Cholera toxin: interaction of subunits with ganglioside GM1. Science 183:656–657
Merchán MA, Collía F, López DE, Saldaña E (1988) Morphology of cochlear root neurons in the rat. J Neurocytol 17:711–725
Mesulam MM (1982) Tracing neuronal connections with horseradish peroxidase. Wiley, New York, pp 1–151
McIlhinney RAJ, Bacon SJ, Smith AD (1988) A simple and rapid method for the production of cholera B-chain coupled to horseradish peroxidase for neuronal tracing. J Neurosci Methods 22:189–194
Osen KK (1969) Cytoarchitecture of the cochlear nuclei in the cat. J Comp Neurol 136:453–484
Osen KK, Mugnaini E, Dahl A-L, Christiansen AH (1984) Histochemical localization of acetylcholinesterase in the cochlear and superior olivary nuclei. A reappraisal with emphasis on the cochlear granule cell system. Arch Ital Biol 122:169–212
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates, 2nd edn. Academic Press, New York
Rajan R (1990) Electrical stimulation of the inferior colliculus at low rates protects the cochlea from auditory desensitization. Brain Res 506:192–204
Robertson D, Winter IM (1988) Cochlear nucleus inputs to olivocochlear neurones revealed by combined anterograde and retrograde labelling in the guinea pig. Brain Res 462:47–55
Rouiller EM, Capt M, Dolivo M, de Ribaupierre F (1989) Neuronal organization of the stapedius reflex pathways in the rat: a retrograde HRP and viral transneuronal tracing study. Brain Res 476:21–28
Sanes DH, Goldstein NA, Ostad M, Hillman DE (1990) Dendritic morphology of central auditory neurons correlates with their tonotopic position. J Comp Neurol 294:443–454
Scheibel ME, Scheibel AB (1974) Neuropil organization in the superior olive of the cat. Exp Neurol 43:339–348
Sofroniew MV (1983) Golgi-like immunoperoxidase staining of neurons producing specific substrates or of neurons transporting exogenous tracer proteins. In: Cuello AC (ed) Immunocytochemistry. Wiley, New York, pp 431–447
Sofroniew MV, Glasmann W (1981) Golgi-like immunoperoxidase staining of hypothalamic magnocellular neurons that contain vasopressin, oxytocin or neurophysin in the rat. Neuroscience 6:619–643
Spangler KM, Henkel CK, Miller IJ Jr (1982) Localization of the motor neurons to the tensor tympani muscle. Neurosci Lett 32:23–27
Stoeckel K, Schwab M, Thoenen H (1977) Role of gangliosides in the uptake and retrograde axonal transport of cholera and tetanus toxin as compared to nerve growth factor and wheat germ agglutinin. Brain Res 132:273–285
Thompson A, Thompson G (1989) Descending auditory projections to olivocochlear neurons in guinea pigs. Assoc Res Otolaryngol 12:344–345
Thompson AM, Thompson GC (1991) Posteroventral cochlear nucleus projections to olivocochlear neurons. J Comp Neurol 303:267–285
Trojanowski JQ, Gonatas JO, Gonatas NK (1981) Conjugates of horseradish peroxidase (HRP) with cholera toxin and wheat germ agglutinin to free HRP as orthogradely transported markers. Brain Res 223:381–385
Vetter DE, Mugnaini E (1989) The lateral olivocochlear system in rats as revealed by cholera toxin-HRP. Soc for Neurosci Abst 15:210
Vetter DE, Mugnaini E (1990) An evaluation of retrograde tracing methods for the identification of chemically distinct cochlear efferent neurons. Arch Ital Biol 128:331–353
Vetter DE, Saldaña E (1990) Descending input from the central nucleus of the inferior colliculus to the medial olivocochlear system in rat: a combined PHA-L and CT-B study. Soc Neurosci Abst 16:716
Vetter DE, Adams JC, Mugnaini E (1991) Chemically distinct rat olivocochlear neurons. Synapse 7:21–43
Wan X-CS, Trojanowski JQ, Gonatas JO (1982) Cholera toxin and wheat germ agglutinin conjugates as neuroanatomical probes, their uptake and clearance, transglangionic and retrograde transport and sensitivity. Brain Res 243:215–224
Warr WB, Guinan JJ, White JS (1986) Organization of the efferent fibers: the lateral and medial olivocochlear systems. In: Altschuler RA, Bobbin RP, Hoffman DW (eds) Neurobiology of hearing: the cochlea. Raven Press, New York, pp 333–348
White JS, Warr WB (1983) The dual origins of the olivocochlear bundle in the albino rat. J Comp Neurol 219:203–214
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Vetter, D.E., Mugnaini, E. Distribution and dendritic features of three groups of rat olivocochlear neurons. Anat Embryol 185, 1–16 (1992). https://doi.org/10.1007/BF00213596
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213596