Summary
Subsequent to the injection of horseradish peroxidase into the parietal eye of adult Lacerta sicula, the course of the parietal nerve and its projections were determined.
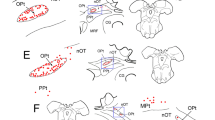
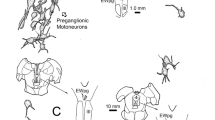
The parietal nerve enters the left habenular ganglion where it branches into a medial and a lateral route. Some nerve fibers decussate within the habenular commissure. Whereas this pathway exhibits a striking asymmetry at the level of the habenular ganglia, its projections to the dorsolateral nucleus of the thalamus, the periventricular hypothalamic area, the preoptic hypothalamic and telencephalic regions, and the pretectal area are arranged in a strictly symmetric manner. A possible innervation of tegmental areas could not be proven due to the presence of endogenous peroxidase within these regions. No parietal nerve fibers were observed in the optic tectum.
In a few animals investigated, scattered labeled perikarya were located in the periventricular hypothalamic gray indicating a parietopetal innervation in Lacerta sicula.
The injection of horseradish peroxidase into one of the lateral eyes revealed terminal areas of the optic nerve within the preoptic region, and the thalamic and pretectal nuclei, displaying partial overlapping with the projections of the parietal nerve to these areas.
From the present investigation further evidence is obtained that the pineal complex of lower vertebrates is a component of the photoneuroendocrine system. Particular emphasis is placed upon the nervous connections between the parietal eye and the hypothalamus, described for the first time in the present study.
Similar content being viewed by others
References
Aron E, Combescot C, Demaret J, Guyon L (1960) Neurosécrétion chez la tortue d'eau Emys leprosa après destruction de la région épiphysaire. CR Acad Sci Paris 251:1914–1916
Berk ML, Heath JE (1975) Effect of preoptic, hypothalamic and telencephalic lesions on thermoregulation in the lizard, Dipsosaurus dorsalis. J Thermal Biol 1:65–78
Braitenberg V, Kemali M (1970) Exceptions to bilateral asymmetry in the epithalamus of lower vertebrates. J Comp Neurol 138:137–146
Brück K, Zeisberger E (1978) Significance and possible central mechanisms of thermoregulatory threshold deviations in thermal adaptation. In: Wang LCH, Hudson JW (eds) Strategies in the cold: natural torpidity and thermogenesis. Academic Press, New York, pp 655–694
Cairney J (1926) A general survey of the forebrain of Sphenodon punctatum. J Comp Neurol 42:255–348
Clausen HJ, Poris EG (1937) The effect of light upon sexual activity in the lizard, Anolis carolinensis, with special reference to the pineal body. Anat Rec 69:39–53
Collin JP (1971) Differentiation and regression of the cells of the sensory line in the epiphysis cerebri. In: Wolstenholme GEW, Knight J (eds) The pineal gland. Churchill Livingstone, Edinburgh London, pp 79–125
Collin JP, Juillard MT, Falcon J (1977) Localization of 5-hydroxytryptaimne and protein(s) in the secretion granules of the rudimentary photoreceptor cells in the pineal of Lacerta. J Neurocytol 6:541–554
Crews D (1979) Neuroendocrinology of lizard reproduction. Biol Reprod 20:51–73
Dodt E (1973) The parietal eye (pineal and parietal organs) of lower vertebrates. In:Jung R (ed) Handbook of sensory physiology. Vol VIII/3B. Springer, Berlin New York, pp 113–140
Dodt E, Scherer E (1968) Photic responses from the parietal eye of the lizard, Lacerta sicula campestris (De Betta). Vision Res. 8:61–72
Eakin RM (1973) The third eye. Univ California Press: Berkeley
Eakin RM, Westfall JA (1959) Fine structure of the retina in the reptilian third eye. J Biophys Biochem Cytol 6:133–134
Ebbesson SOE (1972) A proposal for a common nomenclature for some optic nuclei in vertebrates and the evidence for a common origin of two such cell groups. Brain Behav Evol 6:75–91
Ebbesson SOE (1980) The parcellation theory and its relation to interspecific variability in brain organization, evolutionary and ontogenetic development, and neuronal plasticity. Cell Tissue Res 213:179–212
Edinger L (1899) Untersuchungen über die vergleichende Anatomie des Gehirns. 4. Studien über das Zwischenhirn der Reptilien. Abh Senckenberg Naturforsch Ges 20:161–197
Eldred WD, Finger ThE, Nolte J (1979) Central projections from the frontal organ of Rana pipiens, as demonstrated by the anterograde transport of horseradish peroxidase. Cell Tissue Res 211:215–222
Engbretson GA, Reiner A, Brecha N (1981) Habenular asymmetry and the central connections of the parietal eye of the lizard. J Comp Neurol 198:155–165
Glaser HRS (1958) Increase locomotor activity following shielding of the parietal eye in night lizards. Science 128:1577–1578
Gundy CG, Wurst GZ (1976) The occurrence of parietal eyes in recent Lacertilia. J Herpetol 10:113–121
Gundy CG, Ralph CL, Wurst GZ (1975) Parietal eyes in lizards: zoogeographical correlates. Science 190:671–673
Hafeez MA, Zerihun L (1974) Studies on the central projections of the pineal nerve tract in rainbow trout, Salmo gaidneri Richardson, using cobalt chloride iontophoresis. Cell Tissue Res 154:485–510
Haldar C, Thapliyal JP (1977) Effect of pinealectomy on the annual testicular cycle of Calotes versicolor. Gen Comp Endocrinol 32:395–399
Hamasaki DI (1968) Properties of the parietal eye of the green iguana. Vision Res 8:591–599
Hamasaki DI, Dodt E (1969) Light sensitivity of the lizard's epiphysis cerebri. Pflügers Arch ges Physiol 313:19–29
Hammel HT, Caldwell FT, Abrams RM (1967) Regulation of body temperature in the blue-tongued lizard. Science 156:1260–1262
Hammel HT, Crawshaw LI, Cabanac HP (1973) The activation of behavioral responses in the regulation of body temperature in vertebrates. In: Schonbaum E, Lomax P (eds) The pharmacology of thermoregulation. Karger, Basel, pp 124–141
Hartwig HG (1975) Neurobiologische Studien an photoneuroendokrinen Systemen. Habilitationsschrift. Fachbereich Humanmedizin Giessen.
Itaya SK, Williams TH, Engel EL (1978) Anterograde transport of horseradish peroxidase enhanced by poly-L-oraithine. Brain Res 150:170–176
Kappers JA (1967) The sensory innervation of the pineal organ in the lizard, Lacerta viridis, with remarks on its position in the trend of pineal phylogenetic structural and functional evolution. Z Zellforsch 81:581–618
Kappers JA (1978) Localization of indoleamine and protein synthesis in the mammalian pineal gland. J Neural Transm 13:13–24
Keefer DA (1978) HRP as a retrogradely transported detailed dendritic marker. Brain Res 140:15–32
Kelley DB, Lieberburg I, McEwen BS, Pfaff DW (1978) Autoradiographic and biochemical studies of steroid hormone-concentrating cells in the brain of Rana pipiens. Brain Res 140:287–305
Korf HW, Wagner U (1980) Evidence for a nervous connection between the brain and the pineal organ in the guinea pig. Cell Tissue Res 209:505–510
Korf HW, Zimmermann NH (1980) Der nervöse Apparat im Pinealorgan von Passer domesticus. Verh Anat Ges (in press)
Kuhlenbeck H (1977) The central nervous system of vertebrates. Vol 5/1. Karger, Basel München Paris London New York Sidney
Licht P (1967) Environmental control of annual testicular cycles in the lizard, Anolis carolinensis. I. Interactions of light and temperature in the initiation of testicular recrudescence. J Exp Zool 165:505–516
Licht P, Pearson AK (1970) Failure of parietalectomy to affect the testes in the lizard Anolis carolinensis. Copeia 1970:172–173
Meiniel A, Collin JP, Hartwig HG (1973) Pinéale et troisième oeil de Lacerta vivipara (J), au cours de la vie embryonnaire et postnatale. Étude cytophysiologique des monoamines en microscopie de fluorescence et en microspectrofluorimétrie. Z Zellforsch 144:89–115
Miller WH, Wohbarsht ML (1962) Neural activity in the parietal eye of a lizard. Science 135:316–317
Morgan MJ, O'Donnell JM, Oliver RF (1973) Development of the left-right asymmetry in the habenular nuclei of Rana temporaria. J Comp Neurol 149:203–214
Nowikoff M (1910) Untersuchungen über den Bau, die Entwicklung und die Bedeutung des Parietalauges von Sauriern. Z Wiss Zool 96:118–207
Oksche A (1971) Sensory and glandular elements of the pineal organ. In: Wolstenholme GEW, Knight J (eds) The pineal gland, pp 127–146. Churchill Livingstone, Edinburg London
Oksche A (1976) The neuroanatomical basis of comparative endocrinology. Gen Comp Endocrinol 29:225–239
Oksche A (1978) Pattern of neuroendocrine cell complexes (subunits) in hypothalamic nuclei: neurobiological and phylogenetic concepts. In: Neurosecretion and neuroendocrine activity. Evolution, structure and function. In: Bargmann W, Oksche A, Polenov A, Scharrer B (eds). pp 64–71. Springer, Berlin Heidelberg New York
Oksche A, Kirschstein H (1966) Zur Frage der Sinneszellen im Pinealorgan der Reptilien. Naturwissenschaften 53:46
Oksche A, Kirschstein H (1968) Unterschiedlicher elektronenmikroskopischer Feinbau der Sinneszellen im Parietalauge und im Pinealorgan (Epiphysis cerebri) der Lacertilia. Ein Beitrag zum Epiphysenproblem Z Zellforsch 87:159–192
Oksche A, Hartwig HG (1979) Pineal sense organs — components of photoneuroendocrine systems. Prog Brain Res 52:113–130
Paul E, Zimmermann P (1972) Raktionsmuster verschiedener Mittel- und Zwischenhirnzentren von Rana temporaria L. nach Unterbrechung der Nervenbahnen des Pinealkomplexes. Z Zellforsch 128:512–537
Paul E, Hartwig HG, Oksche A (1971) Neurone und zentralnervöse Verbindungen des Pinealorgans der Anuren. Z Zellforsch 112:466–493
Quay WB (1979) The parietal eye — pineal complex. In: Gans C (ed) Biology of reptiles. Vol 9. Academic Press, London pp 245–406
Ralph CL, Firth BT, Turner JS (1979) The role of the pineal body in ectotherm thermoregulation. Am Zool 19:273–293
Rao Prasada D, Oksche A, Nürnberger F (1981) The structural pattern of lacertilian and ophidian hypothalami and its functional significance. Verh Anat Ges, Varna (in press)
Reiter RJ (1978) The pineal. Vol 3. In: Horrobin DF (ed) Annual Research reviews, pp 1–236. Churchill Livingstone, Edinburgh
Romeis B (1968) Mikroskopische Technik. Oldenbourg, München Wien
Scharrer E (1964) Photo-neuro-endocrine systems: general concepts. Ann N Y Acad Sci 117:13–22
Senn DG (1968) Bau und Ontogenese von Zwischen- und Mittelhirn bei Lacerta sicula (Rafinesque). Acta Anat 71(55):1–150
Simon E, Hammel HT, Oksche A (1977) The thermosensitivity of single units in the rostral brain stem of the conscious Pekin duck. J Neurobiol 8:523–535
Stebbins RC, Eakin RM (1958) The role of the third eye in reptilian behavior. Ann Mus Nov 1870:1–40
Stebbins RC, Cohen NW (1973) The effect of parietalectomy on the thyroid and gonads in free-living Western fence lizards (Sceloporus occidentalis). Copeia 1973:662–668
Studnicka FK (1905) Die Parietalorgane. In: Oppel A (ed) Lehrbuch der vergleichenden mikroskopischen Anatomie der Wirbeltiere. Vol 5. Gustav Fischer, Jena
Ueck M (1979) Innervation of the vertebrate pineal. Prog Brain Res 52:45–88
Underwood H (1977) Circadian organization in lizards: the role of the pineal organ. Science 195:787–589
Vullings HGB (1973) Influence of light and darkness on the hypothalamohypophysial system of Rana esculenta and the involvement of the pineal complex. Z Zellforsch 146:491–500
Zilles K, Nickeleit V (1979) Efferent connections from the brain to the frontal organ in Rana temporaria demonstrated by labeling with horseradish peroxidase. Cell Tissue Res 196:189–192
Author information
Authors and Affiliations
Additional information
Supported by the Deutsche Forschungsgemeinschaft (Grant Ko 758/1)
In partial fulfillment of the requirements of the degree of Dr. med., Faculty of Medicine, Justus Liebig University of Giessen
Rights and permissions
About this article
Cite this article
Korf, H.W., Wagner, U. Nervous connections of the parietal eye in adult Lacerta s. sicula Rafinesque as demonstrated by anterograde and retrograde transport of horseradish peroxidase. Cell Tissue Res. 219, 567–583 (1981). https://doi.org/10.1007/BF00209995
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00209995