Summary
-
1.
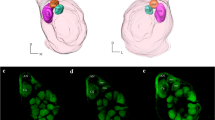
The antennal lobes (ALs) in the brain of the corn earworm moth Helicoverpa zea (formerly Heliothis zea; Lepidoptera: Noctuidae) were examined using combined anatomical and electrophysiological methods. Like other moths, male H. zea possess a sex-specific macroglomerular complex (MGC) for processing information about the female sex-pheromone blend. Unlike other moths, however, the MGC in H. zea consists of 3 distinct glomerular structures: two situated dorsally, and a third situated ventrally (Fig. 1).
-
2.
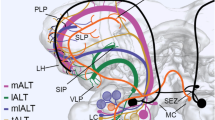
Intracellular recording and staining revealed a population of projection neurons that link the MGC with the protocerebrum (Figs. 4, 7, and 11). Four physiological classes of MGC neurons were identified based upon their responses to stimulation of the antenna with different components of the pheromonal blends of H. zea and Heliothis virescens (a sympatric species). One class responded selectively to the principal component in both species, Z11 16:AL (Figs. 2 and 3). A second, more broadly tuned class showed strong responses to Z11-16:AL and also to other pheromonal components (Figs. 5 and 6). A third class did not respond to Z11-16:AL but did respond to Z9-14:AL, a substance released by H. virescens females that helps attract conspecific males while it inhibits the attraction of H. zea males (Figs. 8, 9 and 10). Some of these neurons also responded to another pheromonal component required for male attraction in H. zea, Z9-16: AL. A fourth class responded in a unique fashion to a blend of Z11-16: AL and Z9-14:AL(Fig. 12).
-
3.
Projection neurons that responded to Z11-16:AL had arborizations in all 3 MGC glomeruli (Figs. 4 and 7), whereas neurons that responded to Z9-14:AL from H. virescens had arborizations in just one of the dorsal glomeruli of the MGC (Fig. 11). Thus these two types of neurons with widely different quality-coding functions have overlapping arborizations in one dorsal glomerulus in the MGC, demonstrating that the MGC is not exclusively involved with processing species-specific (pheromonal) information.
Similar content being viewed by others
Abbreviations
- AL :
-
antennal lobe
- AN :
-
antennal nerve
- Ca :
-
calyces of the mushroom body
- DG :
-
dorsal glomeruli
- FE :
-
female-equivalent
- IACT :
-
inner antenno-cerebral tract
- ILP :
-
inferior lateral protocerebrum
- MGC :
-
macroglomerular complex
- PC :
-
protocerebrum
- VG :
-
ventral glomerulus
References
Almaas TJ, Mustaparta H (1990a) Pheromone reception in the tobacco budworm moth Heliothis virescens. J Chem Ecol 16:1331–1347
Almaas TJ, Mustaparta H (1990b) Heliothis virescens: response characteristics of receptor neurons in sensilla trichodea type 1 and type 2. J Chem Ecol (in press)
Almaas TJ, Christensen TA Mustaparta H (1991) Chemical communication in heliothine moths. I. Antennal receptor neurons encode several features of intra- and interspecific odorants in the male corn earworm moth Helicoverpa zea. J Comp Physiol A
Baker TC (1986) Pheromone-modulated movements of flying moths. In: Payne TL, Birch MC, Kennedy CEJ (eds) Mechanisms in insect olfaction. Oxford University Press, Oxford, pp 39–48
Baker TC (1989) Sex pheromone communication in the Lepidoptera: new research progress. Experientia 45:248–262
Baker TC, Haynes KF (1989) Field and laboratory electroan-tennographic measurements of pheromone plume structure correlated with oriental fruit moth behaviour. Physiol Entomol 14:1–12
Boeckh J, Distler P, Ernst KD, Hösl M, Malun D (1990) Olfactory bulb and antennal lobe. In: Schild D (ed) Chemosensory information processing. Springer, Berlin Heidelberg New York, pp 201–227
Burrows M, Boeckh J, Esslen J (1982) Physiological and morphological properties of interneurones in the deutocerebrum of male cockroaches which respond to female pheromone. J Comp Physiol 145:447–457
Christensen TA, Hildebrand JG (1987a) Functions, organization and physiology of the olfactory pathways in the lepidopteranbrain. In: Gupta AP (ed) Arthropod brain: it's evolution, development, structure and functions. John Wiley and Sons, New York, pp 457–484
Christensen TA, Hildebrand JG (1987b) Male-specific, sex pheromone-selective projection neurons in the antennal lobes of the moth Manduca sexta. J Comp Physiol A 160:553–569
Christensen TA, Hildebrand JG (1988) Frequency coding by central olfactory neurons in the sphinx moth Manduca sexta. Chem Senses 13:123–130
Christensen TA, Geoffrion SC, Hildebrand JG (1990) Physiology of interspecific chemical communication in Heliothis moths. Physiol Entomol 15:275–283
Christensen TA, Mustaparta H, Hildebrand JG (1989a) Discrimination of sex pheromone blends in the olfactory system of the moth. Chem Senses 14:463–477
Christensen TA, Hildebrand JG, Tumlinson JH, Doolittle RE (1989b) The sex pheromone blend of Manduca sexta: responses of central olfactory interneurons to antennal stimulation in male moths. Arch Insect Biochem Physiol 10:281–291
Derby CD, Hamilton KA, Ache BW (1984) Processing of olfactory information at three neuronal levels in the spiny lobster. Brain Res 300:311–319
Erber J, Homberg U, Gronenberg W (1987) Functional roles of the mushroom bodies in insects. In: Gupta AP (ed) Arthropod brain: it's evolution, development, structure and functions. John Wiley and Sons, New York, pp 485–511
Flanagan D, Mercer AR (1989) An atlas and 3-D reconstruction of the antennal lobes in the worker honey bee, Apis mellifera L. (Hymenoptera:Apidae). Int J Insect Morphol Embryol 18:145–159
Grant AJ, O'Connell RJ, Hammond AM Jr (1988) A comparative study of pheromone perception in two species of noctuid moths. J Insect Behavior 1:75–96
Hansson BS, Christensen TA, Hildebrand JG (1991) Functionally distinct subdivisions of the macroglomerular complex in the antennal lobe of the male sphinx moth Manduca sexta. J Comp Neurol (in press)
Hansson BS, Löfstedt C, Löfqvist J (1986) Spatial arrangement of different types of pheromone-sensitive sensilla in a male moth. Naturwissenschaften 73:269–270
Homberg U, Christensen TA, Hildebrand JG (1989) Structure and function of the deutocerebrum in insects. Annu Rev Entomol 34:477–501
Homberg U, Montague RA, Hildebrand JG (1988) Anatomy of antenno-cerebral pathways in the brain of the sphinx moth Manduca sexta. Cell Tissue Res 254:255–281
Kanzaki R, Arbas EA, Hildebrand JG (1991a) Physiology and morphology of protocerebral olfactory neurons of the male moth Manduca sexta. J Comp Physiol A 168:281–298
Kanzaki R, Arbas EA, Hildebrand JG (1991b) Physiology and morphology of descending neurons in pheromone-processing olfactory pathways in the male moth Manduca sexta. J Comp Physiol A 169:1–14
Kanzaki R, Arbas EA, Strausfeld NJ, Hildebrand JG (1989) Physiology and morphology of projection neurons in the antennal lobe of the male moth Manduca sexta. J Comp Physiol A 165:427–453
Kehat M, Dunkelblum E (1990) Behavioral responses of male Heliothis armigera (Lepidoptera: Noctuidae) moths in a flight tunnel to combinations of components identified from female sex pheromone glands. J Insect Behavior 3:75–83
Klun JA, Bierl-Leonhardt BA, Plimmer JR, Sparks AN, Primiani M, Chapman OL, Lepone G, Lee GH (1980a) Sex pheromone chemistry of the female tobacco budworm moth, Heliothis virescens. J Chem Ecol 6:177–183
Klun JA, Plimmer JR, Bierl-Leonhardt BA, Sparks AN, Primiani M, Chapman OL, Lee GH, Lepone G (1980b) Sex pheromone chemistry of female corn earworm moth, Heliothis zea. J Chem Ecol 6:165–175
Koontz MA, Schneider D (1987) Sexual dimorphism in neuronal projections from the antennae of silk moths (Bombyx mori, Antheraea polyphemus) and the gypsy moth (Lymantria dispar). Cell Tissue Res 249:39–50
Linn CE, Campbell MG, Roelofs WL (1986) Male moth sensitivity to multicomponent pheromones: Critical role of female-released blend in determining the functional role of components and active space of the pheromone. J Chem Ecol 12:659–668
Linn CE, Campbell MG, Roelofs WL (1987) Pheromone components and active spaces: what do moths smell and where do they smell it? Science 237:650–652
Lucas P, Renou M (1989) Responses to pheromone compounds in Mamestra suasa (Lepidoptera: Noctuidae) olfactory neurones. J Insect Physiol 35:837–845
Masson C, Mustaparta H (1990) Chemical information processing in the olfactory system of insects. Physiol Rev 70:199–245
Matsumoto SG, Hildebrand JG (1981) Olfactory mechanisms in the moth Manduca sexta: response characteristics and morphology of central neurons in the antennal lobes. Proc R Soc Lond 213:249–277
Olberg RM (1983) Pheromone-triggered flip-flopping interneurons in the ventral nerve cord of the silkworm moth, Bombyx mori. J Comp Physiol 152:297–307
Olberg RM, Willis MA (1990) Pheromone-modulated optomotor response in male gypsy moths, Lymantria dispar L.: directionally selective visual interneurons in the ventral nerve cord. J Comp Physiol A 167:707–714
Pope MM, Gaston LK, Baker TC (1982) Composition, quantification, and periodicity of sex pheromone gland volatiles from individual Heliothis virescens females. J Chem Ecol 8:1043–1055
Pope MM, Gaston LK, Baker TC (1984) Composition, quantification, and periodicity of sex pheromone volatiles from individual Heliothis zea females. J Insect Physiol 30:943–945
Raina AK, Stadelbacher EA, Ridgway RL (1989) Comparison of sex pheromone composition and pheromone-mediated male behavior of laboratory-reared and wild Heliothis zea (Lepidoptera:Noctuidae). J Chem Ecol 15:1259–1265
Roelofs WL, Hill AS, Cardé RT, Baker TC (1974) Two sex pheromone components of the tobacco budworm moth, Heliothis virescens. Life Sci 14:1555–1562
Rospars JP (1983) Invariance and sex-specific variations of the glomerular organization in the antennal lobes of a moth, Mamestra brassicae, and a butterfly Pieris brassicae. J Comp Neurol 220:80–96
Rospars JP (1988) Structure and development of the insect antennodeutocerebral system. Int J Insect Morphol Embryol 17:243–294
Teal PEA, Tumlinson JH, McLaughlin JR, Heath R, Rush RA (1984) (Z)-11-hexadecen-1-ol: a behavioural modifying chemical present in the pheromone gland of female Heliothis zea (Lepidoptera: Noctuidae). Can Entomol 116:777–779
Teal PEA, Tumlinson JH, Heath RR (1986) Chemical and behavioural analyses of volatile sex pheromone components released by calling Heliothis virescens (F.) females (Lepidoptera: Noctuidae). J Chem Ecol 12:107–126
Tumlinson JH, Hendricks PE, Mitchell ER, Doolittle RE, Brennan MM (1975) Isolation, identification and synthesis of the sex pheromone of the tobacco budworm. J Chem Ecol 1:203–214
Vetter RS, Baker TC (1984) Behavioural responses of male Heliothis zea moths in sustained-flight tunnel to combinations of 4 compounds identified from female sex pheromone gland. J Chem Ecol 10:193–202
Vickers NJ, Christensen TA, Mustaparta H, Baker TC (1991) Chemical communication in heliothine moths. III. Flight behavior of male Helicoverpa zea and Heliothis virescens in response to varying ratios of intra- and interspecific sex pheromone components. J Comp Physiol A 169:275–280
Waldrop B, Christensen TA, Hildebrand JG (1987) GABAmediated synaptic inhibition of projection neurons in the antennal lobes of the sphinx moth, Manduca sexta. J Comp Physiol A 161:23–32
Wigglesworth VB (1957) The use of osmium in the fixation and staining of tissues. Proc R Soc Lond B 147:185–199
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Christensen, T.A., Mustaparta, H. & Hildebrand, J.G. Chemical communication in heliothine moths. J Comp Physiol A 169, 259–274 (1991). https://doi.org/10.1007/BF00206990
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00206990