Summary
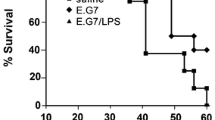
Liposomes composed of chemically synthesized glyceroglycolipids, such as 1,2-dipalmityl-[β-cellobiosyl-(1′ → 3)]-glycerol (Cel-DAG), 1,2-dipalmityl-[β-lactosyl-(1′ → 3)]-glycerol, or 1,2-dipalmityl-[β-maltosyl-(1′ → 3)]-glycerol, were found to enhance protective immunity against transplantable tumor cells (sarcoma 180) in ICR mice. Peritoneal exudate cells prepared from mice treated in vivo with Cel-DAG showed cytostatic activity in vitro against the mouse leukemia cell line, EL-4. Adherent cells separated from this preparation showed similar activity. Peritoneal cells from polypeptone-injected mice acquired appreciable cytostatic activity when incubated in vitro in the presence of glyceroglycolipid liposomes. The adherent cell fraction alone showed rather weak cytostatic activity when pretreated with the glyceroglycolipids, and full activity was restored by supplementing with the nonadherent cell fraction. The ability of glycolipids to induce tumoricidal effects was affected by cholesterol content: with increasing cholesterol content, the activities decreased. Cholesterol-free glycolipid liposomes were taken more efficiently by macrophages than cholesterol-containing liposomes. Cholersterol modifies the surface property of glyceroglycolipid liposomes. Activation of macrophages is responsible for enhancement of protective immunity against tumor cells by injection of these glycolipids in vivo.
Similar content being viewed by others
References
Chihara G, Maeda Y, Hamuro J, Sasaki T, Fukuoka F (1969) Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes. Nature 222:687
Dennert G, Tucker D (1973) Antitumor polysaccharide lentinan — a T cell adjuvant. J Natl Cancer Inst 51:1727
Endo T, Inoue K, Nojima S (1982) Physical properties and barrier functions of synthetic glyceroglycolipids. J Biochem 92:953
Endo T, Nojima S, Inoue K (1982) Reactivity of sphingolipid haptens in glyceroglycolipid liposomes as well as glycerophospholipid liposomes. In: New vistas in glycolipid research. Plenum Publishing Corporation, New York, pp 353
Fidler IJ, Darnell JH, Budmen MB (1976) Tumoricidal properties of mouse macrophages activated with mediators from rat lymphocytes stimulated with concanavalin A. Cancer Res 36:3608
Gley I, Davies P, Derr J, Krett N, Barranger JA (1981) Relationship between production and release of lymphocyte-activating factor (Interleukin 1) by murine macrophages. I. Effects of various agents. Cell Immunol 64:293
Gounaris K, Barber J (1983) Monogalactosyldiacylglycerol: the most abundant polar lipid in Nature. Trends Biochem Sci 8:378
Hamuro J, Maeda YY, Arai Y, Fukuoka F, Chihara G (1971) The significance of the higher structure of the polysaccharides lentinan and pachymaran with regard to their antitumor activity. Chem Biol Interact 3:69
Hamuro J, Wagner H, Röllinghoff M (1978) β(1 → 3)Glucans as a probe for T cell specific immune adjuvants. II. Enhanced in vitro generation of cytotoxic T lymphocytes. Cell Immunol 38:328
Hamuro J, Röllinghoff M, Wagner H (1980) Induction of cytotoxic peritoneal exudate cells by T-cell immune adjuvants of the β (1 → 3) glucan-type lentinan and its analogues. Immunology 39:551
Hoppe CA, Lee YC (1983) The binding and processing of mannose-bovine serum albumin derivatives by rabbit alveolar macrophages. J Biol Chem 258:14193
Inoue K (1974) Permeability properties of liposomes prepared from dipalmitoyllecithin, dimyristoyllecithin, egg lecithin, rat liver lecithin and beef brain sphingomyelin. Biochim Biophys Acta 339:390
Ishizuka I, Yamakawa T (1985) Glycoglycerolipids. In: Glycolipids. Elsevier, Amsterdam, pp 101
Iwamoto K, Sunamoto J, Inoue K, Endo T, Nojima S (1982) Liposomal membranes. XV Importance of surface structure in liposomal membranes of glyceroglycolipids. Biochim Biophys Acta 691:44
Kate M, Chan TH, Stanacev NZ (1963) Aliphatic diether analogs of glyceride-derived lipids. I. Synthesis of D-α, β-dialkyl glyceryl ethers. Biochemistry 2:394
Kumagai K, Itoh K, Hinuma S, Tada M (1979) Pretreatment of plastic petri dishes with fetal calf serum. A simple method for macrophage isolation. J Immunol Methods 29:17
Meltzer MS (1981) Macrophage activation for tumor cytotoxicity: characterization of priming and trigger signals during lymphokine activation. J Immunol 127:179
Okuda T, Yoshioka Y, Ikekawa T, Chihara G, Nishioka K (1972) Anticomplementary activity of antitumor polysaccharides. Nature 238:59
Pidgeon C, Schreiber RD, Schultz RM (1983) Macrophage activation: Synergism between Hybridoma MAF and poly(I)poly(C) delivered by liposomes. J Immunol 131:311
Schreiber RO, Altman A, Katz DH (1982) Identification of a T-cell hybridoma which produces extraordinary quantities of macrophage activating factor. J Exp Med 156:677
Schultz RM (1982) Synergistic activation of macrophages by lymphokines and lipopolysaccharides: evidence for lymphokine as the primer and interferon as the trigger. J Interferon Res 2:459
Sone S, Fidler IJ (1980) Synergistic activation by lymphokines and muramyl dipeptide of tumoricidal properties in rat alveolar macrophages. J Immunol 125:2454
Struck DK, Pagano RE (1980) Insertion of fluorescent phospholipids into the plasma membrane of a mammalian cell. J Biol Chem 255:5404
Tsushima S, Yoshioka Y, Tanida S, Nomura H, Hojima S, Hozumi M (1982) Synthesis and antimicrobial activities of alkyl lysophospholipids. Chem Pharm Bull 30:3260
Wu P, Tin GW, Baldeschwieler JD (1981) Phagocytosis of carbohydrate-modified phospholipid vesicles by macrophages. Proc Natl Acad Sci USA 78:2033
Author information
Authors and Affiliations
Additional information
This work was supported in part by Grants-in-Aid (Nos. 58010010, and 59870076) for Scientific Research from the Ministry of Education, Science and Culture of Japan
Rights and permissions
About this article
Cite this article
Naito, M., Kudo, I., Mukai-Sato, Y. et al. Activation of mouse peritoneal macrophages by synthetic glyceroglycolipid liposomes. Cancer Immunol Immunother 24, 158–164 (1987). https://doi.org/10.1007/BF00205594
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00205594