Summary
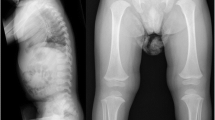
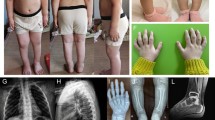
We have examined the procollagens and collagens produced by skin fibroblasts from a patient with Ehlers-Danlos syndrome type VII. The patient was heterozygous for an abnormal α2(I) chain migrating with the approximate size of pNα2(I) chains after pepsin digestion. Peptide mapping suggested that the abnormality was located at the amino-terminus of the α2(I) chain. Quantitative analysis of the α2(I) mRNA indicated loss of the exon 6 sequences, and subsequent polymerase chain reaction amplification of cDNA demonstrated a deletion of the 54 bp of exon 6 from some of the α2(I) mRNA. Analysis of genomic DNA from the patient revealed a single base change in one COL1A2 allele, substituting an A for a G as the first base of intron 6. This change mutates the obligate GT-dinulceotide splicing signal to AT and leads to exon skipping with splicing from exon 5 to exon 7. Loss of exon 6 sequences results in the loss of the procollagen-N-propeptidase cleavage site and a lysine residue that normally participates in covalent intermolecular crosslinking within collagen fibres.
Similar content being viewed by others
References
Barsh GS, Petersen KF, Byers PH (1981) Peptide mapping of collagen chains using CNBr cleavage of proteins within polyacrylamide gels. Coll Relat Res 1:543–548
Beighton P, DePaepe A, Danks D, Finidori G, Gedde-Dahl T, Goodman R, Hall JG, Hollister DW, Horton W, McKusick VA, Opitz JM, Pope FM, Pyeritz RE, Rimoin DL, Sillence D, Spranger JW, Thompson E, Tsipouras P, Viljoen D, Winship I, Young I (1988) International Nosology of Heritable Disorders of Connective Tissue, Berlin 1986. Am J Med Genet 29:581–594
Bonadio J, Byers PH (1985) Subtle structural alterations in the chains of type I procollagen produce osteogenesis imperfecta type II. Nature 316:363–366
Cole WG, Chan D, Chambers GW, Walker ID, Bateman JF (1986) Deletion of 24 amino acids from the proα1(I) chain of type I procollagen in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem 261:5496–5503
Eyre DR, Shapiro FD, Aldridge JF (1985) A heterozygous collagen defect in a variant of the Ehlers-Danlos syndrome type VII. Evidence for a deleted amino-telopeptide domain of the proα2(I) chain. J Biol Chem 260:11322–11329
Kuivaniemi H, Sabol C, Tromp G, Sippola-Thiele M, Prockop DJ (1988) A 19-base pair deletion in the proα2(I) gene of type I procollagen that causes in frame RNA splicing from exon 10 to exon 12 in a proband with atypical osteogenesis imperfecta and in his asymptomatic mother. J Biol Chem 263:11407–11413
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lapiere CM, Lenaers A, Kohn LD (1971) Procollagen peptidase: an enzyme excising the coordination peptides of procollagen. Proc Natl Acad Sci USA 68:3054–3058
Lenaers A, Ansay M, Nusgens BV, Lapiere CM (1971) Collagen made of extended α-chains, procollagen in genetically-defective dermatosparaxic calves. Eur J Biochem 23:533–543
Lichtenstein JR, Martin GR, Kohn LD, Byers PH, McKusick VA (1973) Defective conversion of procollagen to collagen in a form of Ehlers-Danlos syndrome. Science 182:298–300
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbour Laboratory, Cold Spring Harbor, NY, pp 191–193
Marvit J, DiLella AG, Brayton K, Ledley FD, Robson KJH, Woo SLC (1987) GT to AT transition at a splice donor site causes exon skipping of the preceding exon. Nucleic Acids Res 15:5613–5628
McKusick VA (1972) Heritable disorders of connective tissue, 4th edn. Mosby, St Louis, MO
Nicholls AC, De Paepe A, Narcisi P, Dalgleish R, De Keyser F, Matton M, Pope FM (1988) Likage of a polymorphic marker for the type III collagen gene (COL3A1) to atypical autosomal dominant Ehlers-Danlos syndrome type IV in a large Belgian pedigree. Hum Genet 78:276–281
Padgett RA, Grabowski PJ, Konarska MM, Seiler S, Sharp PA (1986) Splicing of messenger RNA precursors. Annu Rev Biochem 55:1119–1150
Saiki RK, Scharf S, Faloona F, Mullis KB, Horn GT, Erlich HA, Arnheim N (1985) Enzymatic amplification of β-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230:1350–1354
Sanger F, Nicklin S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Steinmann B, Tuderman L, Peltonen L, Martin GR, McKusick VA, Prockop DJ (1980) Evidence for a structural mutation of procollagen type I in a patient with the Ehlers-Danlos syndrome type VII. J Biol Chem 255:8887–8893
Treisman R, Proudfoot NJ, Shander M, Maniatis T (1982) A single base change at a splice site in a β∘-thalassemic gene causes abnormal RNA splicing. Cell 29:903–911
Tromp G, Prockop DJ (1988) Single base mutation in the proα2(I) collagen gene that causes efficient splicing of RNA from exon 27 to exon 29 and synthesis of a shortened but in frame proα2(I) chain. Proc Natl Acad Sci USA 85:5254–5258
Tsipouras P, Myers JC, Ramirez F, Prockop DJ (1983) Restriction fragment length polymorphism associated with the proα2(I) gene of human type I procollagen. J Clin Invest 72:1262–1267
Weil D, Bernard M, Combates N, Wirtz MK, Hollister DW, Steinmann B, Ramirez F (1988) Identification of a mutation that causes exon skipping during collagen pre-mRNA splicing in an Ehlers-Danlos syndrome variant. J Biol Chem 263:8561–8564
Weil D, D'Alessio M, Ramirez F, Wet W de, Cole WG, Chan D, Bateman JF (1989a) A base substitution in the exon of a collagen gene causes alternative splicing and generates a structurally abnormal polypeptide in a patient with Ehlers-Danlos syndrome type VII. EMBO J 8:1705–1710
Weil D, D'Alessio M, Ramirez F, Steinmann B, Wirtz MK, Glanville RW, Hollister DW (1989b) Temperature-dependent expression of a collagen splicing defect in the fibroblasts of a patient with Ehlers-Danlos syndrome type VII. J Biol Chem 264:16804–16809
Wet W de, Bernard M, Benson-Chanda V, Chu M-L, Dickson L, Weil D, Ramirez F (1987) Organization of the human proα2(I) collagen gene. J Biol Chem 262:16032–16036
Wirtz MK, Glanville RW, Steinmann B, Rao V, Hollister DW (1987) Ehlers-Danlos syndrome type VIIB. Deletion of 18 amino acids comprising the N-telopeptide region of a proα2(I) chain. J Biol Chem 262:16376–16385
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nicholls, A.C., Oliver, J., Renouf, D.V. et al. Ehlers-Danlos syndrome type VII: a single base change that causes exon skipping in the type I collagen α2(I) chain. Hum Genet 87, 193–198 (1991). https://doi.org/10.1007/BF00204180
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00204180